Fragmentation of pentacoordinate spirobicyclic aminoacylphosphoranes (P-AAs) by electrospray ionization tandem mass spectrometry concerning P-O and P-N bond cleavage
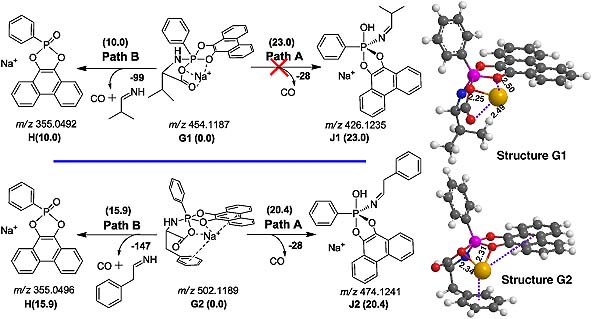
The fragmentation pathways of both protonated and sodiated pentacoordinate spirobicyclic aminoacylphosphoranes (P-AAs) have been studied by electrospray ionization multi-stage mass spectrometry (ESI-MSn) in positive mode. The possible pathways and their mechanisms are elucidated through the combination of ESI-MS/MS, isotope (15 N and 2H) labeling and high-resolution Fourier transform ion cyclotron resonance (FTICR)-MS/MS. The relative Gibbs free energies (ΔG) of the product ions and possible fragmentation pathways are estimated at the B3LYP/6-31 G(d) level of theory. The theoretical calculations show that both protonated and sodiated P-AAs would quickly fragment before Berry pseudorotation. For protonated P-AAs, they have different tendencies to P–O or P–N bond cleavage. For sodiated P-AAs, the P–N bond is easier to cleave and produces the tetracoordinated phosphorus ion H. These results to some extent may give a clue to the chemistry of the active sites of phosphoryl transfer enzymes and will enrich the gas-phase ESI-MS ion chemistry of pentacoordinate phosphoranes.
