A metallanaphthalyne complex from zinc reduction of a vinylcarbyne complex
Submitted by Jun Zhu on Fri, 11/01/2013 - 00:02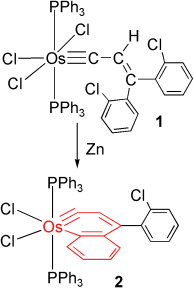
Cl prevents insertion: The first metallanaphthalyne 2 has been obtained by Zn reduction of Os carbyne complex 1. The key to its isolation was the use of o-chlorophenyl instead of phenyl substituents to avoid formation of a putative hydrido metallanaphthalyne intermediate (supported by DFT calculations), which undergoes migratory insertion of the carbyne into the OsH bond and rearrangement to give an indenyl complex as the final product.