Nature Chemistry


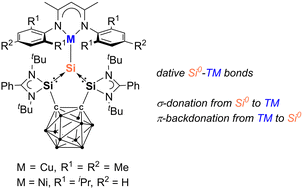
The first silylone-3d-metal complexes, LSiCu(NacNacM) (2) [L = 1,2-(RSi)2-1,2-C2B10H10, R = PhC(NtBu)2; NacNacM = HC(CMeNMes)2, Mes = 2,4,6-Me3-C6H2] and LSiNi(NacNacD) (3) [NacNacD = HC(CMeNDipp)2, Dipp = 2,6-iPr2-C6H3], are reported, resulting from the reaction of the strongly σ-donating and chelating bis(silylenyl)-ortho-carborane silylone LSi0 with [(NacNacMCu)2benzene] and [(NacNacDNi)2toluene], respectively.
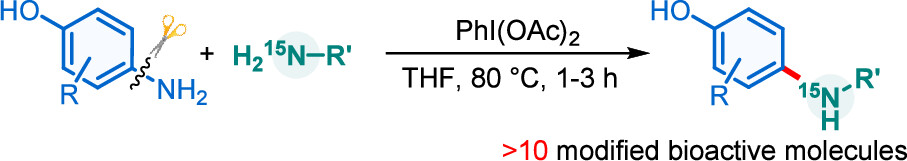
Activation of the aryl C–N bond underpins critical challenges in modern organic synthesis. Herein, the direct amination of anilines is presented via hypervalent iodine-mediated transient dearomatized phenolate intermediates, enabling selective C(aryl)–NH2 bond cleavage under mild conditions. A library of bioactive p-alkylaminophenols is synthesized in up to 85% yields within 3 h. Being used in late-stage drug diversification and mechanistic studies, this protocol offers a modular platform for complex amine construction.
Learning from nature has emerged as a promising strategy for catalyst development, wherein the remarkable performance of catalysts selected by nature over billions of years of evolution serves as a basis for the creative design of high-performance catalysts. Hydrogenases, with their exceptional catalytic activity in hydrogen oxidation and production, have been employed as prototypes for human learning to achieve better catalyst design. A comprehensive understanding of hydrogenases' structures and catalytic mechanisms is crucial to replicate and exceed their performance.
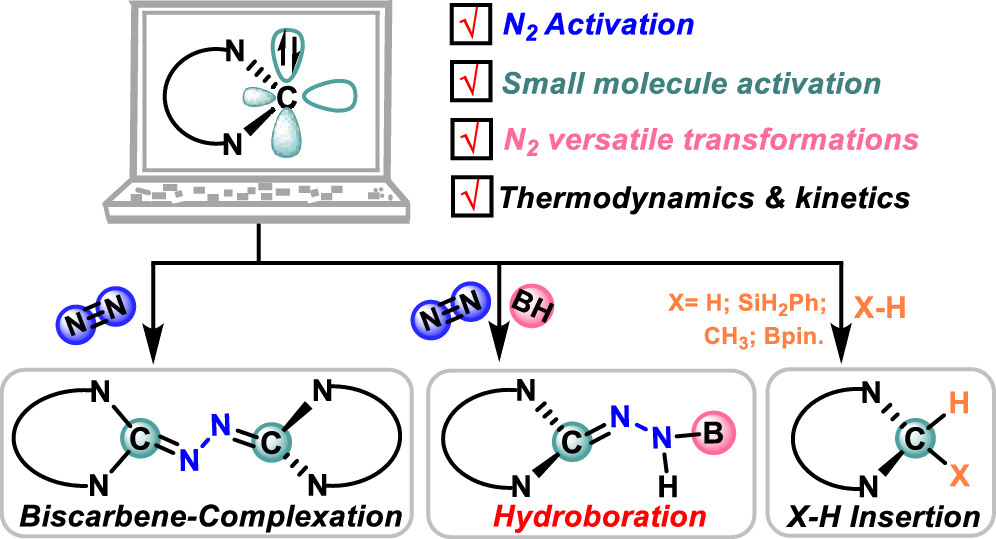
Although the carbene-catalyzed N2 fixation process had been investigated by scientists for decades prior to borylene species, the interest in the carbene-mediated N2 activation process has drawn less attention than that of borylene species in the past few years, especially unique σ0π2 carbenes. Herein, we demonstrate the important role of unique σ0π2 carbenes in the 1,1-hydroboration and bis-carbene functionalization of N2 using density functional theory calculations.
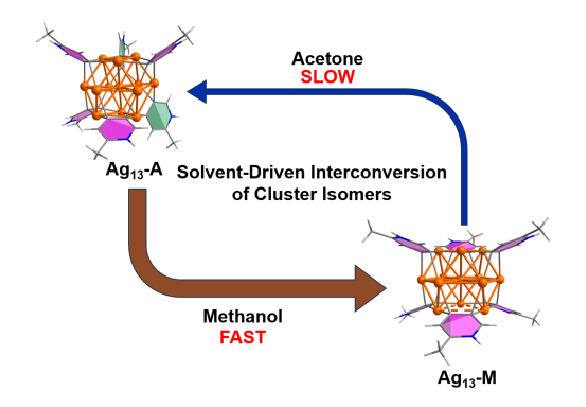
Solvent screening is pivotal in the optimization of reaction conditions for metal-catalyzed reactions. However, to date little is known about the role of solvent effects in driving in situ structural evolution of metal-containing species towards polynuclear organometallic cluster intermediates and determining their reactivity. We herein isolate two distinct thirteen-membered silver cluster isomers, Ag13-A and Ag13-M, in acetone and methanol, respectively.
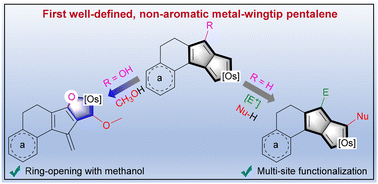
Since the concept of aromaticity was first introduced in transition metal complexes, metals have become a crucial component for modulating aromaticity, leading to a variety of structural frameworks. Initial studies successfully achieved aromatic pentalene dianions through metal-ion coordination, and recent advancements in bridgehead metallapentalenes have demonstrated the transformation of antiaromaticity into aromaticity.
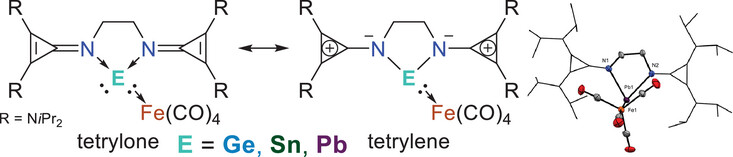
We report on the utilization of the ethylene-bridged bis[(dialkylamino)cyclopropenimine] (bisCPI) ligand, LCPI, to give access to new main-group E(II) halide complexes (E = Ge, Sn, Pb; 1, 2, 3). Subsequent reduction with Collman's reagent (Na2Fe(CO)4 • dioxane) enables the isolation of a series of zero-valent tetrylone-tetracarbonyl iron complexes, (LCPI)E(Fe(CO)4 (E = Ge (4), Sn (5), Pb (6)).
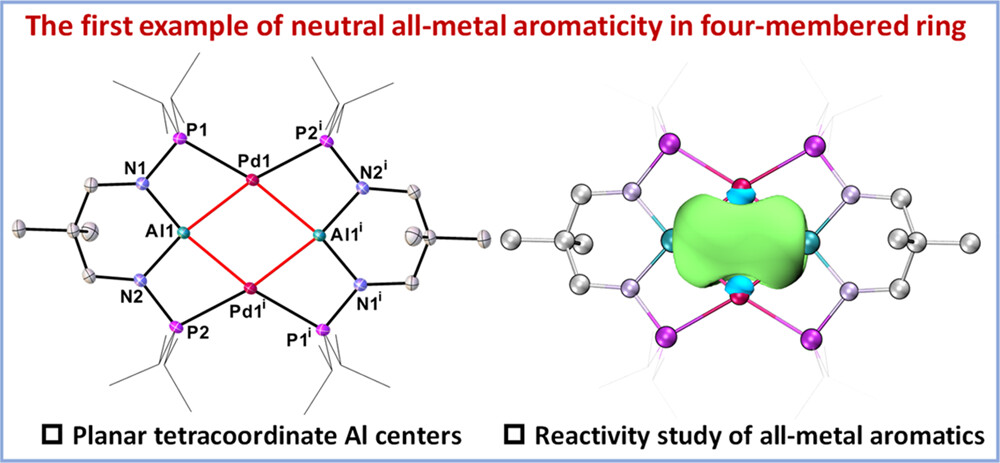
Aromaticity is a cornerstone concept in chemistry, playing a crucial role in understanding molecular stability and reactivity. Traditionally, aromaticity has been primarily associated with cyclic planar conjugated organic molecules composed solely of carbon, but it has recently expanded to include metal-containing systems. However, metal-only aromatics remain extremely scarce. Here, we present the first neutral all-metal aromatic cluster with a rhombic geometry.

Recent research has sparked significant interest in exploring the effects of BN unit doping on the electronic structure of isoelectronic and isostructural benzene analogs, driven by their promising applications in pharmaceuticals and material sciences. In this study, we provide the first comprehensive investigation of BN/CC isosterism in 2,5-dihydro-1,4,2,5-diazadiborinine and its isomers (1−13) through density functional theory (DFT) calculations and machine learning-based analysis.
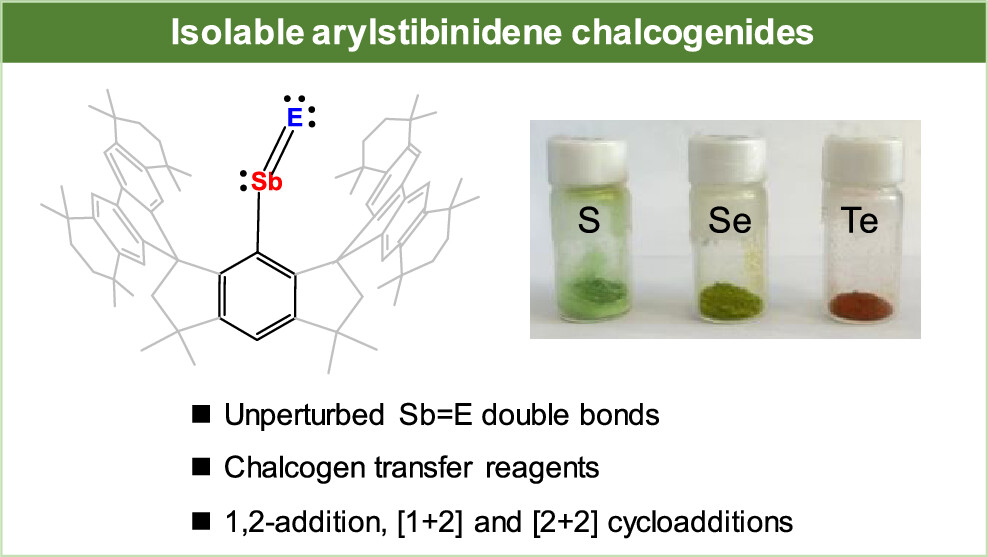
Nitroso compounds, R-N═O, containing N═O double bonds are ubiquitous and widely utilized in organic synthesis. In contrast, heavier congeners of nitroso compounds, namely pnictinidene chalcogenides R-Pn = E (Pn = P, As, Sb, Bi; E = O, S, Se, Te), are highly reactive and scarce. They have been stabilized in the coordination sphere of Lewis acid/base or by pronounced contribution from resonance structures, whereas free species with unperturbed pnictogen-chalcogen double bonds remains elusive.
Copyright © 2025,
Theme Originally Created by Devsaran
