Nature Chemistry




Dinitrogen activation under mild conditions is important but extremely challenging due to the inert nature of the N-N triple bond evidenced by high bond dissociation energy (945 kJ/mol) and large HOMO-LUMO gap (10.8 eV). In comparison with largely developed transition metal systems, the reported main group species on dinitrogen activation are rare. Here, we carry out density functional theory calculations on methyleneboranes to understand the reaction mechanisms of their dinitrogen activation.

White phosphorus (P4) undergoes degradation to P2 moieties if exposed to the new N,N-bis(silylenyl)aniline PhNSi2 1 (Si=Si[N(tBu)]2CPh), furnishing the first isolable 2,5-disila-3,4-diphosphapyrrole 2 and the two novel functionalized Si=P doubly bonded compounds 3 and 4. The pathways for the transformation of the non-aromatic 2,5-disila-3,4-diphosphapyrrole PhNSi2P2 2 into 3 and 4 could be uncovered.

Using the potentially tridentate N,N’-bis(N-heterocyclic silylene)pyridine [SiNSi] pincer-type ligand, 2,6-N,N’-diethyl-bis[N,N’-di-tert-butyl(phenylamidinato)silylene] diaminopyridine, led to the first isolable bis(silylene)pyridine-stabilized manganese(0) complex, {к3-[SiNSi]Mn(dmpe)} 4 (dmpe = (Me2P)2C2H4), which represents an isolobal 17 VE analogue of the elusive Mn(CO)5 radical.
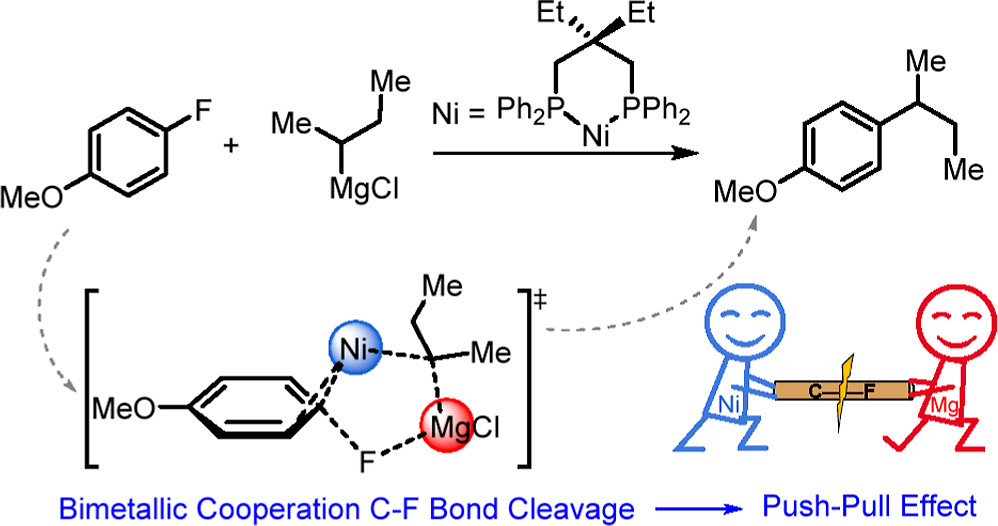
The Ni-catalyzed Kumada–Tamao–Corriu (KTC) cross-coupling between aryl fluorides and alkyl Grignard reagents has been used to achieve a highly selective Csp2–Csp3 bond construction via the carbon–fluorine (C–F) bond activation. However, the detailed mechanism of this groundbreaking KTC reaction remains unclear. Herein, we perform a series of analyses by density functional theory (DFT) calculations in order to understand the reaction mechanisms for the selective activation of a highly inert C–F bond by Ni catalysts with bidentate phosphorus ligands.
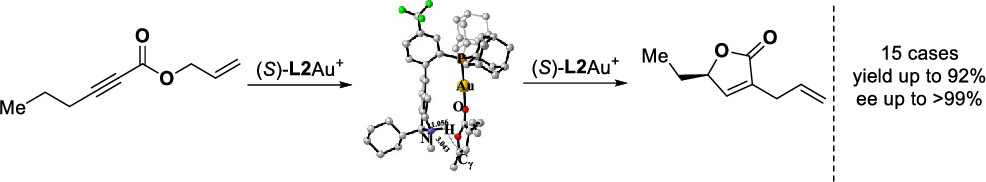
A highly efficient construction of chiral γ-substituted α-allyl-α,β-butenolides with up to >99% enantiomeric excess from readily available allylic ynoates is realized. In this asymmetric gold catalysis, the cationic gold(I) catalyst featuring a bifunctional phosphine ligand enables a four-step cascade which permits the conversion of a diverse array of allylic ynoates into valuable chiral α,γ-disubstituted α,β-butenolides.
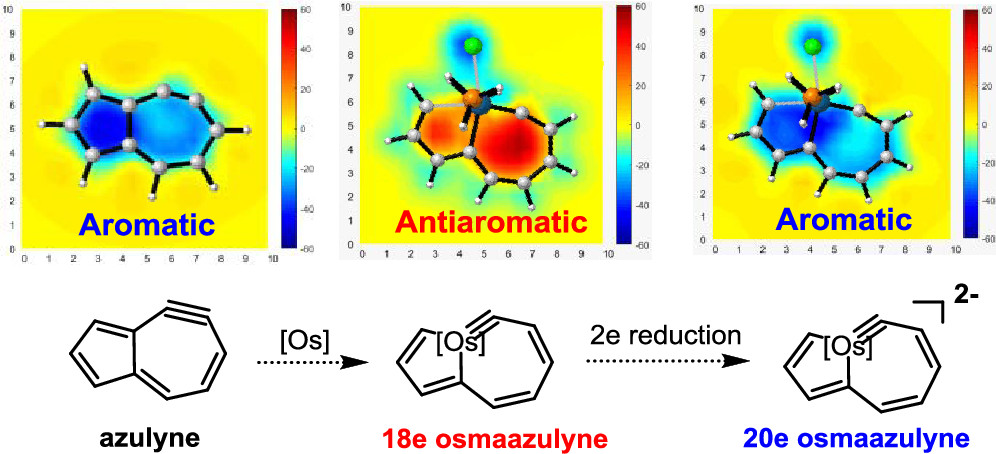
The 18-electron rule states that metal complexes with 18 valence electron metal centers are thermodynamically stable because nine valence orbitals of transition metals including one s orbital, three p orbitals, and five d orbitals can collectively accommodate 18 electrons, achieving the same electron configuration as the noble gas in the period. Thus, 20-electron compounds are extremely rare due to a violation of such a rule.
Activation of atmospherically abundant dinitrogen (N2) under mild conditions has been a great challenge in chemistry for decades because of the significantly strong N≡N triple bond. The traditional strategy of N2 activation was mainly limited to metallic species until the ground-breaking achievement of N2 activation by two-coordinated borylenes was achieved experimentally in 2018. On the other hand, carborane derivatives have attracted considerable interest for small-molecule activation. Still, the utilization of carborane derivatives in N2 activation remains elusive.

Activation of thermodynamically stable and kinetically inert dinitrogen (N2) has been a great challenge due to a significantly strong triple bond. Recently, the experimental study on the N2 activation by boron species, a highly reactive two-coordinated borylene, broke through the limitation of traditional strategy of N2 activation by metal species. Still, the study on metal-free N2 activation remains undeveloped.
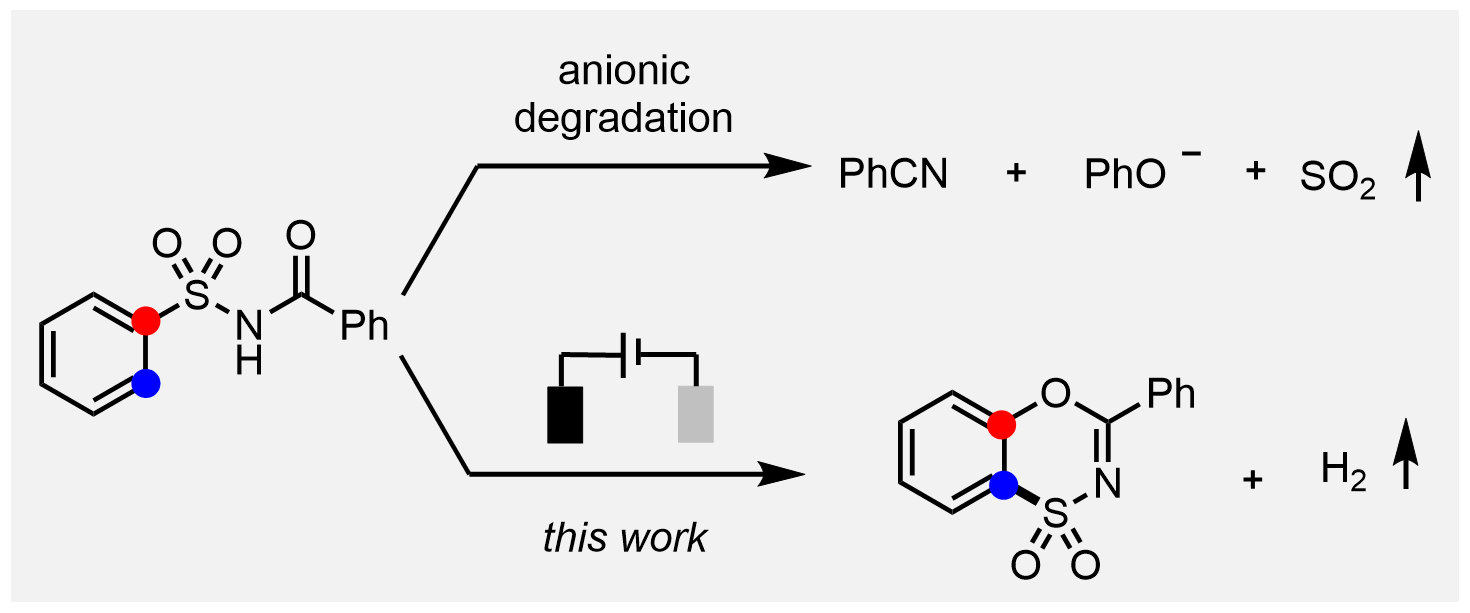
Benzoxathiazine dioxide, as a bioisostere of the clinically widely used diazoxide, exhibits interesting biological activity. However, limited success has been achieved in terms of its concise and direct synthesis. We report herein a facile electrochemical migratory cyclization of N -acylsulfonamides to access a diverse array of benzoxathiazine dioxides. The inclusion of electrochemistry is crucial for realizing such a novel transformation, which is substantiated both by the experiments and density-functional-theory calculations.

New types of metal-free white phosphorus (P4) activation are reported. While the phosphine-silylene-substituted dicarborane 1, CB-SiP {CB = ortho-C,C´-C2B10H10, Si = PhC(tBuN)2Si, P = P[N(tBu)CH2]2}, activates white phosphorus in a 2:1 molar ratio to yield the P5-chain containing species 2, the analogous bis(silylene)-substituted compound 3, CB-Si2, reacts with P4 in the molar ratio of 2:1 to furnish the first isolable 1,3-diphospha-2,4-disilabutadiene (Si=P-Si=P-containing) compound 4.
Copyright © 2026,
Theme Originally Created by Devsaran
