Predicting Dinitrogen Activation by Five-Electron Boron-Centered Radicals
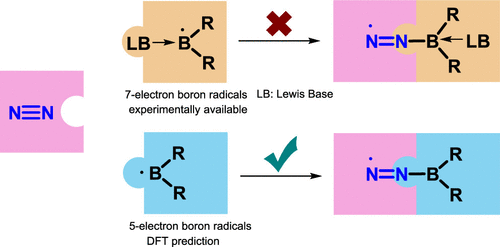
Due to the high bond dissociation energy (945 kJ mol–1) and the large highest occupied molecular orbital–lowest unoccupied molecular orbital (HOMO–LUMO) gap (10.8 eV), dinitrogen activation under mild conditions is extremely challenging. On the other hand, the conventional Haber–Bosch ammonia synthesis under harsh conditions consumes more than 1% of the world’s annual energy supply. Thus, it is important and urgent to develop an alternative approach for dinitrogen activation under mild conditions. In comparison with transition metals, main group compounds are less explored for nitrogen activation. Here, we carry out density functional theory calculation to screen boron radicals for dinitrogen activation. As a result, the experimentally available seven-electron boron-centered radicals are found to be inactive to N2 activation, whereas some five-electron boron-centered radicals become favorable for dinitrogen activation, inviting experimental chemists’ examination. The principal interacting spin–orbital analyses suggest that a five-electron boron-centered radical can mimic a transition metal on a synergic interaction with dinitrogen in the transition states.
