In Search of Flexible Molecular Wires with Near Conformer-Independent Conjugation and Conductance: A Computational Study
Submitted by Jun Zhu on Fri, 03/07/2014 - 00:17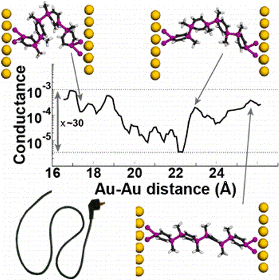
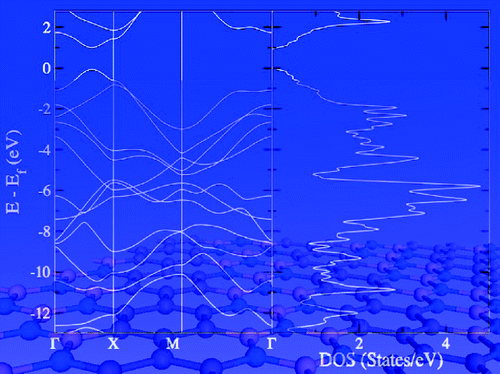
Oligomers of 1,4-disila/germa/stannacyclohexa-2,5-dienes as well as all-carbon 1,4-cyclohexadienes connected via E—E single bonds (E = C, Si, Ge, or Sn) were studied through quantum chemical calculations in an effort to identify conformationally flexible molecular wires that act as molecular “electrical cords” having conformer-independent conjugative and conductive properties. Our oligomers display neutral hyperconjugative interactions (σ/π-conjugation) between adjacent σ(E—E) and π(C═C) bond orbitals, and these interactions do not change with conformation.