Formation of covalent Ga–C bonds on liquid metal nanoparticles with enhanced stability and anti-oxidation
Submitted by Jun Zhu on Wed, 10/29/2025 - 09:25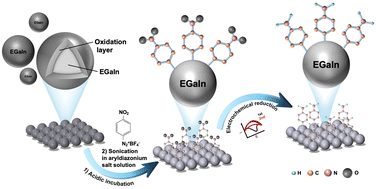
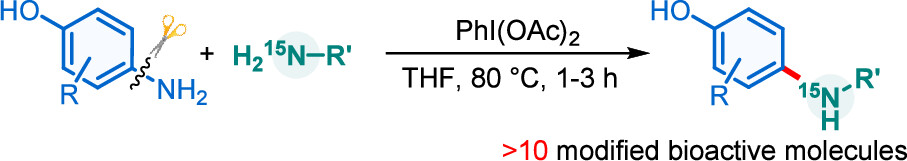
Surface modification of Eutectic Gallium Indium (EGaIn) to inhibit oxidation has been a long-term challenge in materials science, with limited research reporting the formation of a covalent bond between Ga and modifiers for stability purposes. Taking advantage of the strong reductive properties of EGaIn, this study developed a simple method for spontaneous reduction of aryldiazonium salts on the EGaIn nanoparticle surface to form stable covalent Ga–C sigma bonds, effectively suppressing surface oxidation.