Reactivity of Nickel Cations Liganded by Acetylacetone and Benzoylacetone with N2: Organic Ligand Effects
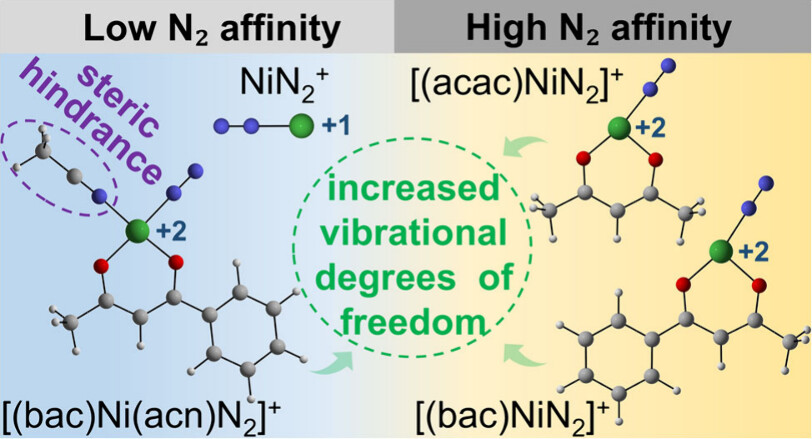
N2 adsorption on metal sites under mild conditions and its dependence on the chemical environment are crucial for understanding the fundamental mechanism and designing an efficient catalyst. Here, electrospray ionization mass spectrometry (ESI-MS) experiments reveal distinct differences in N2 binding capabilities among nickel complexes: both [(acetylacetonato)Ni]+ and [(benzoylacetonato)Ni]+ gas-phase cations exhibit high N2 adsorption efficiency, while the bare Ni+ and [(benzoylacetone)Ni(acetonitrile)]+ cations show negligible reactivity toward N2. Density functional theory calculations reveal that organic ligands modulate the nickel oxidation state from +1 in the isolated Ni+ to +2 in organometallic complexes, thereby enhancing the electrophilicity of the metal center and promoting N2 chemisorption. The stabilization of N2 adsorption products is further driven by (1) the formation of stable coordination bonds and (2) increased vibrational degrees of freedom, which facilitate thermal energy redistribution. In addition, steric hindrance from the acetonitrile ligand significantly suppresses the N2 adsorption. Frontier orbital analysis confirms the σ-donation from the lone pairs of N2 to unoccupied Ni-d orbitals. This work advances the understanding of the synergistic effects between organic ligands and transition metals in N2 adsorption and activation.
