An Isotope (O-18, N-15, and H-2) Technique to Investigate the Metal Ion Interactions Between the Phosphoryl Group and Amino Acid Side Chains by Electrospray Ionization Mass Spectrometry
Submitted by Jun Zhu on Fri, 11/01/2013 - 01:04
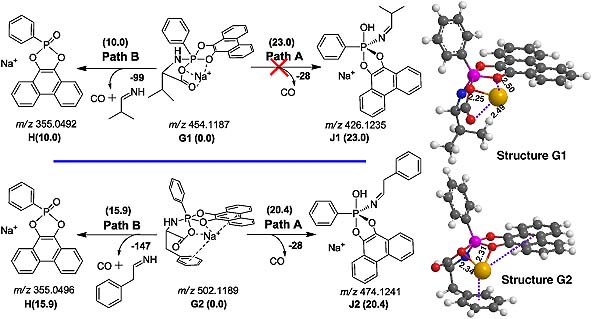
Cationic metal ion-coordinated N-diisopropyloxyphosphoryl dipeptides (DIPP-dipeptides) were analyzed by electrospray ionization multistage tandem mass spectrometry (ESI-MS n ). Two novel rearrangement reactions with hydroxyl oxygen or carbonyl oxygen migrations were observed in ESI-MS/MS of the metallic adducts of DIPP-dipeptides, but not for the corresponding protonated DIPP-dipeptides.