An Isotope (O-18, N-15, and H-2) Technique to Investigate the Metal Ion Interactions Between the Phosphoryl Group and Amino Acid Side Chains by Electrospray Ionization Mass Spectrometry
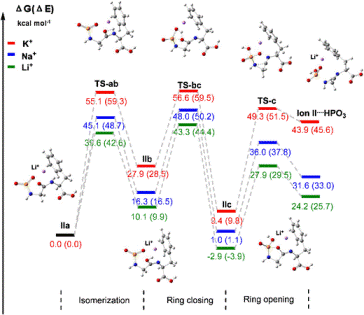
Cationic metal ion-coordinated N-diisopropyloxyphosphoryl dipeptides (DIPP-dipeptides) were analyzed by electrospray ionization multistage tandem mass spectrometry (ESI-MS n ). Two novel rearrangement reactions with hydroxyl oxygen or carbonyl oxygen migrations were observed in ESI-MS/MS of the metallic adducts of DIPP-dipeptides, but not for the corresponding protonated DIPP-dipeptides. The possible oxygen migration mechanisms were elucidated through a combination of MS/MS experiments, isotope (18O, 15N, and 2H) labeling, accurate mass measurements, and density functional theory (DFT) calculations at the B3LYP/6-31 G(d) level. It was found that lithium and sodium cations catalyze the carbonyl oxygen migration more efficiently than does potassium and participation through a cyclic phosphoryl intermediate. In addition, dipeptides having a C-terminal hydroxyl or aromatic amino acid residue show a more favorable rearrangement through carbonyl oxygen migration, which may be due to metal cation stabilization by the donation of lone pair of the hydroxyl oxygen or aromatic π-electrons of the C-terminal amino acid residue, respectively. It was further shown that the metal ions, namely lithium, sodium, and potassium cations, could play a novel directing role for the migration of hydroxyl or carbonyl oxygen in the gas phase. This discovery suggests that interactions between phosphorylated biomolecules and proteins might involve the assistance of metal ions to coordinate the phosphoryl oxygen and protein side chains to achieve molecular recognition.
