Reactivity of Nickel Cations Liganded by Acetylacetone and Benzoylacetone with N2: Organic Ligand Effects
Submitted by Jun Zhu on Mon, 08/11/2025 - 20:22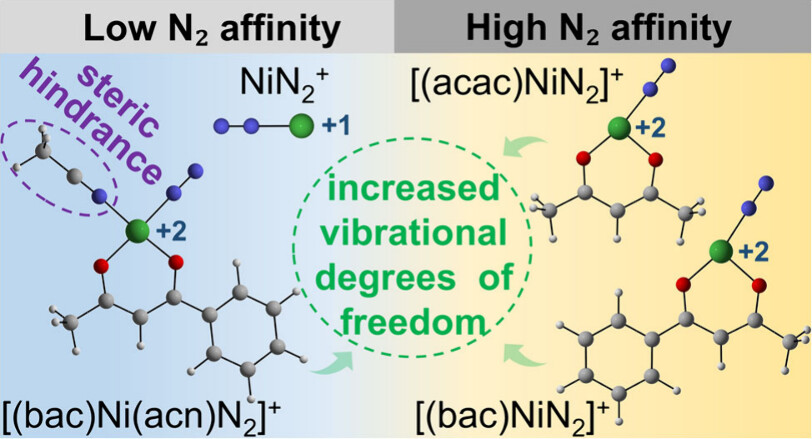
N2 adsorption on metal sites under mild conditions and its dependence on the chemical environment are crucial for understanding the fundamental mechanism and designing an efficient catalyst. Here, electrospray ionization mass spectrometry (ESI-MS) experiments reveal distinct differences in N2 binding capabilities among nickel complexes: both [(acetylacetonato)Ni]+ and [(benzoylacetonato)Ni]+ gas-phase cations exhibit high N2 adsorption efficiency, while the bare Ni+ and [(benzoylacetone)Ni(acetonitrile)]+ cations show negligible reactivity toward N2.


