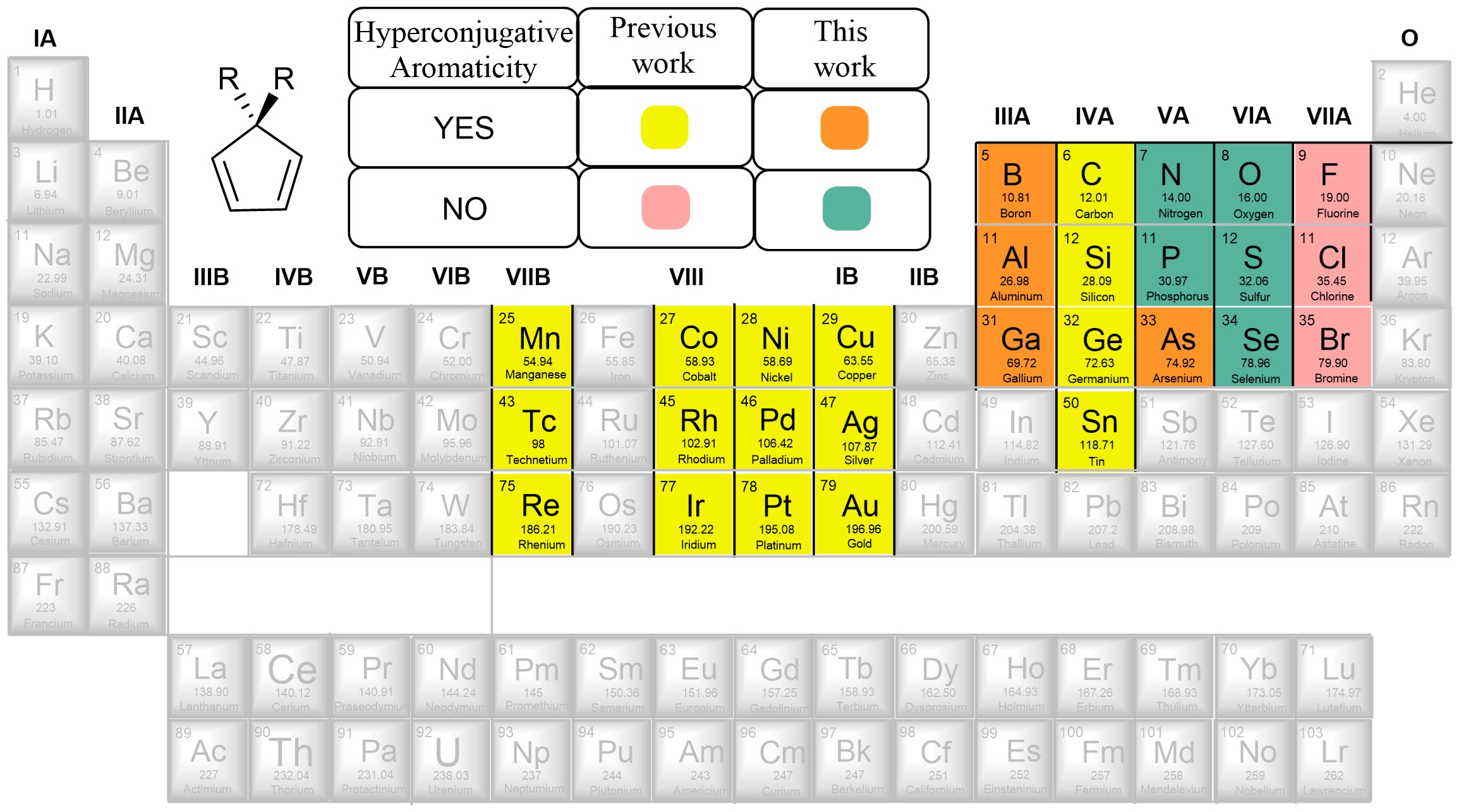
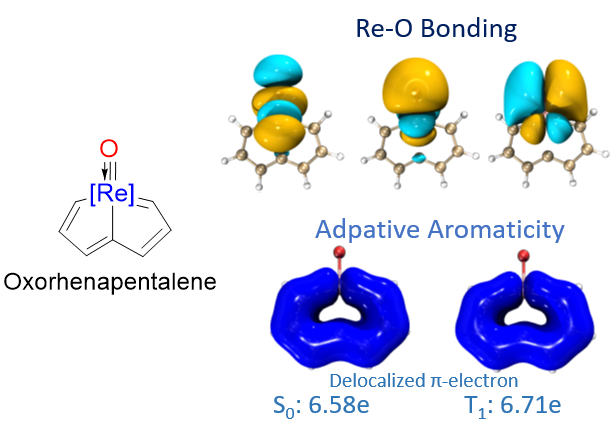
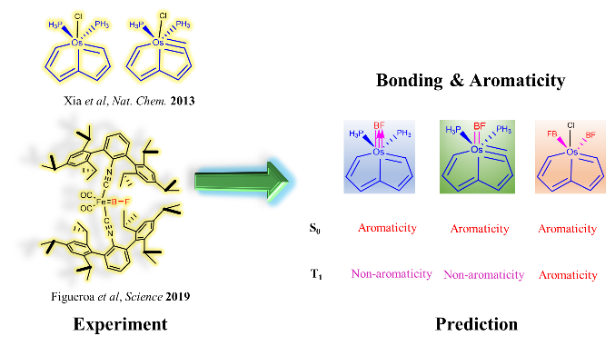
NO-induced adaptive aromaticity in furan, thiophene and selenophene
Submitted by Jun Zhu on Sun, 04/13/2025 - 20:44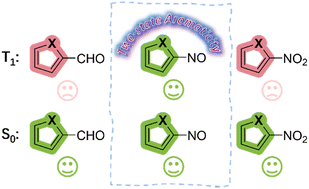
The concept of adaptive aromaticity, which denotes two-state aromaticity in both the lowest singlet and triplet states, stands in marked contrast to the traditional one-state aromaticity governed by the Hückel and Baird rules. Nonetheless, organic compounds exhibiting adaptive aromaticity remain particularly rare.