Synthesis of Five-Membered Osmacycloallenes and Conversion into Six-Membered Osmacycloallenes
Submitted by Jun Zhu on Sun, 11/24/2013 - 20:07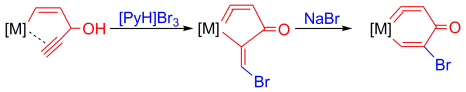
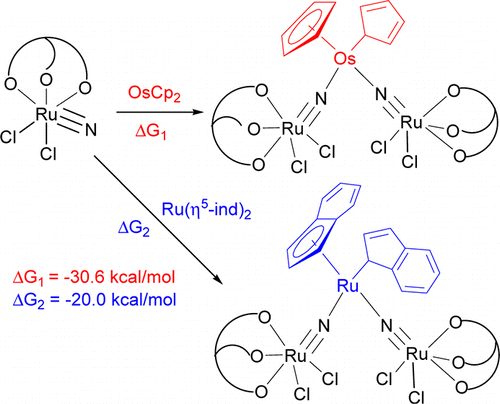
Highly stable five-membered metallacycloallenes were synthesized under mild conditions. Calculations revealed that the incorporation of transition-metal moieties relieves considerable strain and indicates a trend toward ring enlargement in the five-membered metallacycloallenes. Conversion into six-membered metallacycloallenes was confirmed experimentally.