Interconversion of Metallanaphthalynes and Indenylidene Complexes: A DFT Prediction
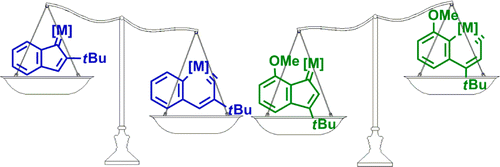
Metallaaromatics have attracted considerable interest of both theoretical and experimental chemists. However, there have been only two metallanaphthalynes isolated so far. Thus, developing new synthetic approaches is urgent. Here we present thorough density functional theory (DFT) calculations on the thermodynamics and kinetics of the isomerization between metallanaphthalynes and metal indenylidene complexes. The effects of metal centers, ligands, and substituents on the metallabicycles were examined systematically. Our results reveal that, in comparison with the third-row transition metals, the second-row metals have a tendency to form metal indenylidenes rather than metallanaphthalynes. In addition, π-donor and π-acceptor ligands can stabilize the metallanaphthalynes and indenylidene complexes, respectively. Steric effects at the meta position also play a role in the stabilization of metallanaphthalynes. Electron-donating groups (EDGs) at the meta position and second-row transition metals give lower barriers in comparison with electron-withdrawing groups (EWGs) and third-row transition metals. Finally, an interconversion of osmanaphthalynes and indenylidene complexes was achieved by simply tuning the substituents on the metallabicycles. Therefore, our findings open an avenue to metal indenylidene complexes and metallanaphthalynes.
