Stable Iso-osmabenzenes from a Formal [3+3] Cycloaddition Reaction of Metal Vinylidene with Alkynols
Submitted by Jun Zhu on Fri, 11/01/2013 - 00:22
Authors:
Qianyi Zhao, Lei Gong, Chunfa Xu, Jun Zhu*, Xumin He, Haiping Xia*
Journal:
Angew. Chem. Int. Ed.
Year:
2011
Volume:
50
FirstPage-LastPage:
1354–1358
TOC:

Abstract:
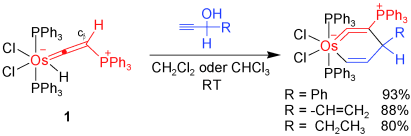
The magic of Os: An unprecedented formal [3+3] cycloaddition reaction of 1 with alkynols affords stable iso-osmabenzenes at room temperature (see scheme). The phosphonium substituent at the Cβ position and the 18e− nature of the compound play key roles in the origin of the high thermal stability of the products. Isomerization of iso-osmabenzenes into η5-cyclopentadienyl complexes through metalated cyclopentadiene intermediates is also described.
Doi:
10.1002/ange.201006442
