Isolation of an Eleven‐Atom Polydentate Carbon‐Chain Chelate Obtained by Cycloaddition of a Cyclic Osmium Carbyne with an Alkyne
Submitted by Jun Zhu on Sat, 09/01/2018 - 16:20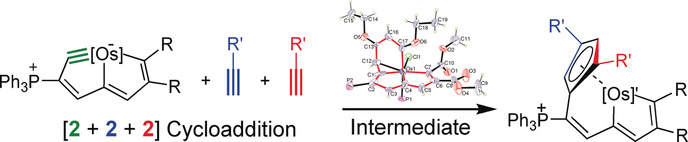
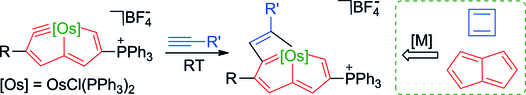
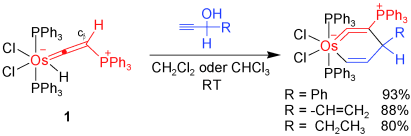
Carbon ligands have long played an important role in organometallic chemistry. However, previous examples of all‐carbon chelating ligands are limited. Herein, we present a novel complex with an eleven‐atom carbon chain as a polydentate chelating ligand. This species was formed by the [2+2+2] cycloaddition reaction of two equivalents of an alkyne with an osmapentalyne that contains the smallest carbyne bond angle (127.9°) ever observed. Density functional calculations revealed that electron‐donating groups play a key role in the stabilization of this polydentate carbon‐chain chelate.