Nature Chemistry




The Pd-catalyzed Buchwald–Hartwig coupling reaction is important in the construction of the C-N bond due to various applications in organic synthesis. Quantum chemical calculations are widely used in understanding reaction mechanisms whereas the machine learning method is extremely popular in recognizing the relationships of data. Here, we combine density functional theory calculations with the support vector regression method to probe the origin of the higher efficiency of terphenyl phosphine ligand over the biaryl counterpart in the Buchwald–Hartwig C-N coupling reaction.

Singlet fission (SF) presents an attractive solution to overcome the Shockley–Queisser limit of single-junction solar cells. The conversion from an initial singlet state to final triplet is mediated by the correlated triplet pair state 1(T1T1). Despite significant advancement on 1(T1T1) properties and its role in SF, a comprehensive understanding of the energetic landscape during SF is still unclear.
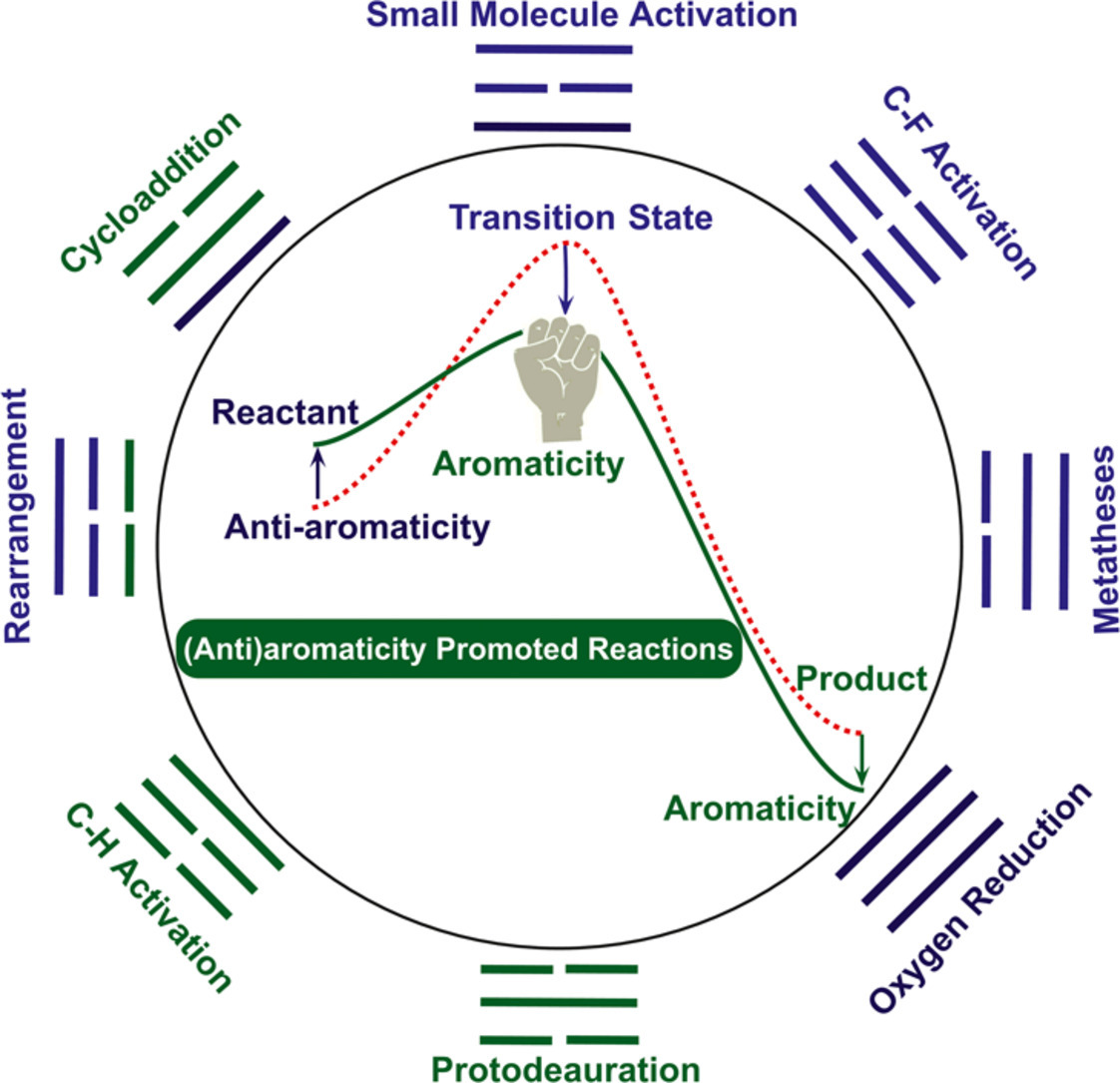
Aromaticity, in general, can promote a given reaction by stabilizing a transition state or a product via a mobility of π electrons in a cyclic structure. Similarly, such a promotion could be also achieved by destabilizing an antiaromatic reactant. However, both aromaticity and transition states cannot be directly measured in experiment. Thus, computational chemistry has been becoming a key tool to understand the aromaticity-driven reaction mechanisms.
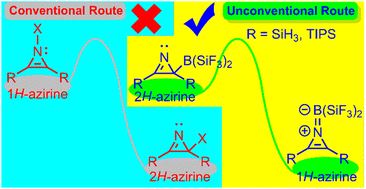
1H-azirine, a highly reactive, antiaromatic, and unstable tautomer of the aromatic, stable, and (sometimes) isolable 2H-azirine, is stabilized, both thermodynamically and kinetically, via an unprecedented route, where the latter serves as the precursor–exploiting electronic and steric elements. Our density functional theory results invite experimentalists to realize isolable 1H-azirine.

Visible-light-mediated dearomatisation of pyrroles is a powerful strategy for the synthesis of pharmaceuticals and bioactive compounds. Herein we present the chemiluminescent reaction between pyrrole metalated-Ir(III) complex [Ir(K2C,N-DPP)(H)(Cl)(PPh3)2] (1) and singlet oxygen to form a ketoamide complex [Ir(K2C,N-ketoamide)(H)(Cl)(PPh3)2] (2). Complex 2 are fully characterized by NMR and single crystal X-ray diffraction analysis.

Open-shell molecules with unpaired electrons and a high-spin S ≥ 1 configuration are of fundamental importance in chemistry, biology and molecular electronics. Among metal-free systems, carbon- and silicon-based triplet diradicals with two unpaired electrons and strong ferromagnetic coupling are proposed as key intermediates in many organic and organometallic transformations but their isolation remains challenging due to their very high reactivity. Here we report the facile synthesis of isolable 1,3-disilapyrroles which act as organosilicon-based delocalized triplet diradicals.
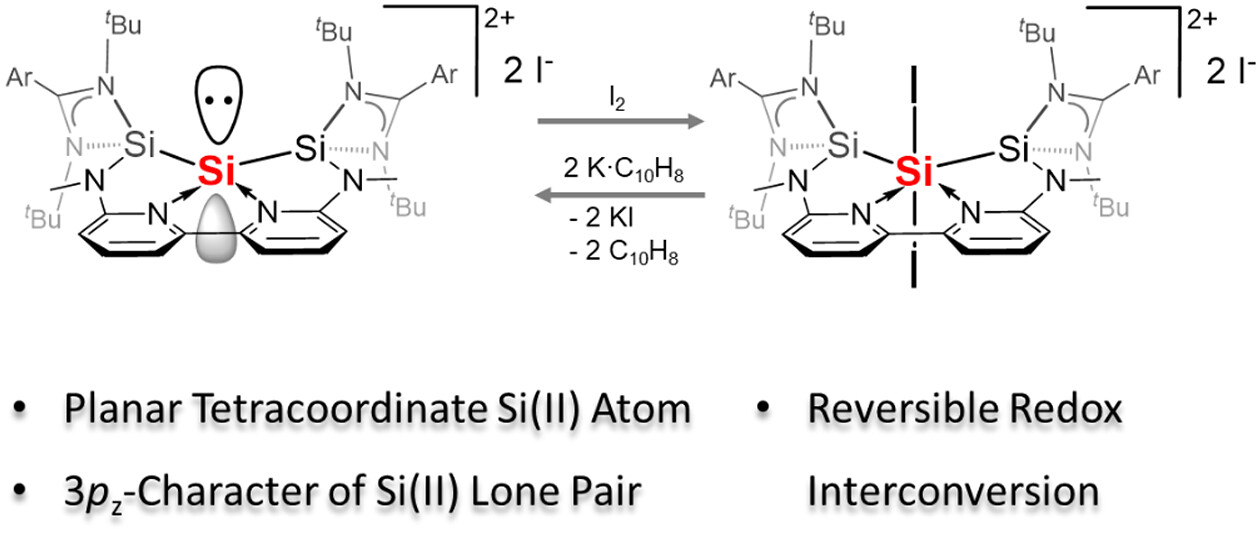
For a long time, planar tetracoordinate carbon (ptC) represented an exotic coordination mode in organic and organometallic chemistry, but it is now a useful synthetic building block. In contrast, realization of planar tetracoordinate silicon (ptSi), a heavier analogue of ptC, is still challenging. Herein we report the successful synthesis and unusual reactivity of the first ptSi species of divalent silicon present in 3, supported by the chelating bis(N-heterocyclic silylene)bipyridine ligand, 2,2′-{[(4-tBuPh)C(NtBu)]2SiNMe}2(C5N)2, 1].

Antiaromaticity is extended from aromaticity as a complement to describe the unsaturated cyclic molecules with antiaromatic destabilization. To prepare antiaromatic species is a particularly challenging goal in synthetic chemistry because of the thermodynamic instability of such molecules. Among that, both Hückel and Möbius antiaromatic species have been reported, whereas the Craig one has not been realized to date. Here, we report the first example of planar Craig antiaromatic species.
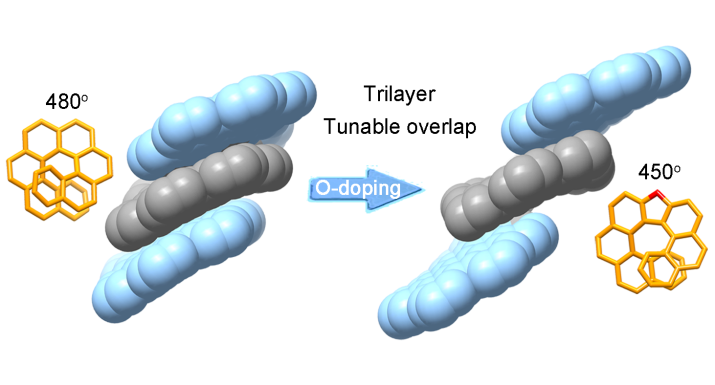
The synthesis of well-defined nanocarbon multilayers, beyond the bilayer structure, is still a challenging goal. Herein, two trilayer nanographenes were synthesized by covalently linking nanographene layers through helicene bridges. The structural characterization of the trilayer nanographenes revealed a compact trilayer-stacked architecture.
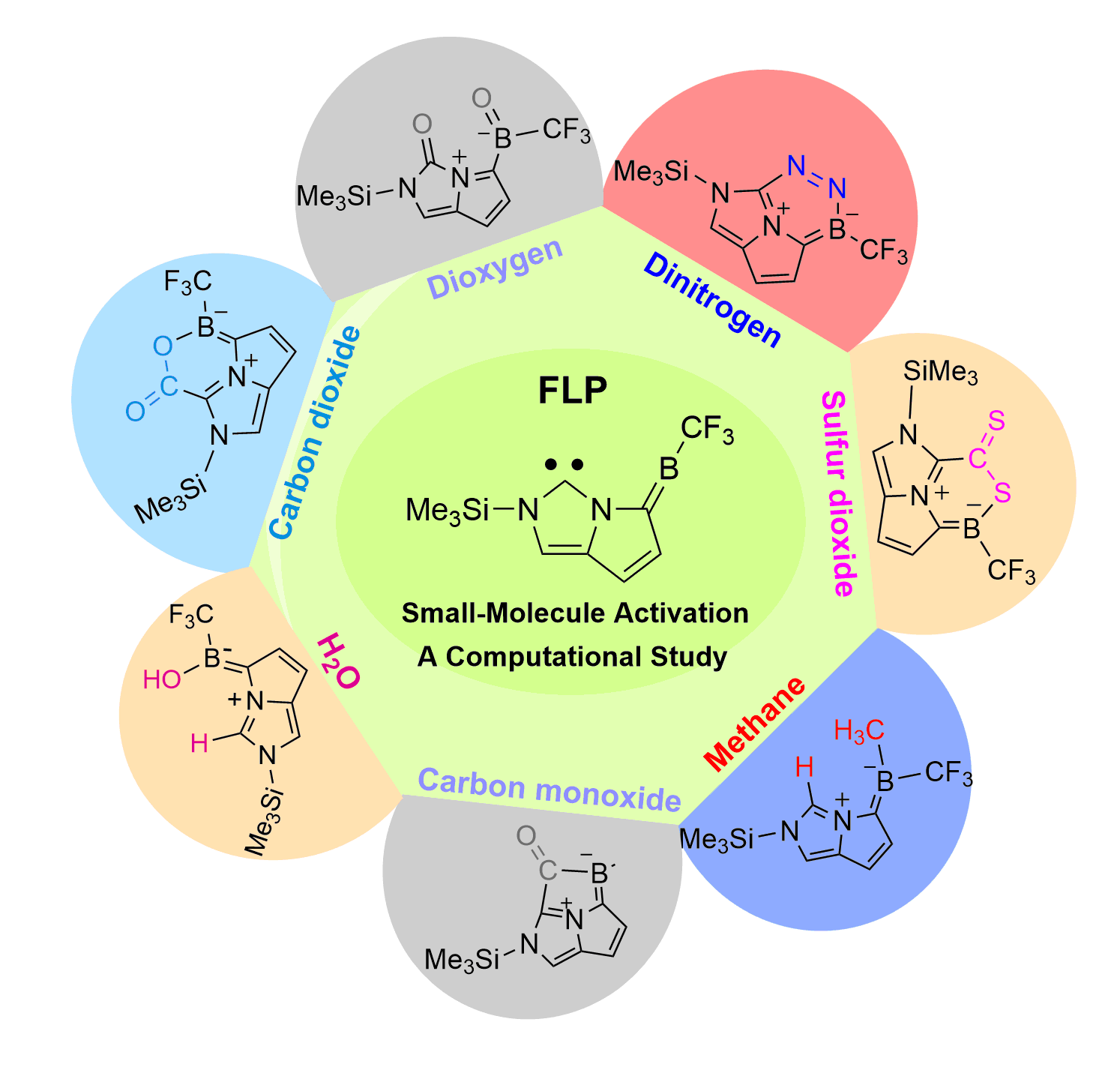
Dinitrogen (N2) activation is particularly challenging under ambient conditions because of its large highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO-LUMO) gap (10.8 eV) and high bond dissociation energy (945 kJ mol–1) of the NΞN triple bond, attracting considerable attention from both experimental and theoretical chemists. However, most effort has focused on metallic systems. In contrast, nitrogen activation by frustrated Lewis pairs (FLPs) has been initiated recently via theoretical calculations.
Copyright © 2026,
Theme Originally Created by Devsaran
