Nature Chemistry



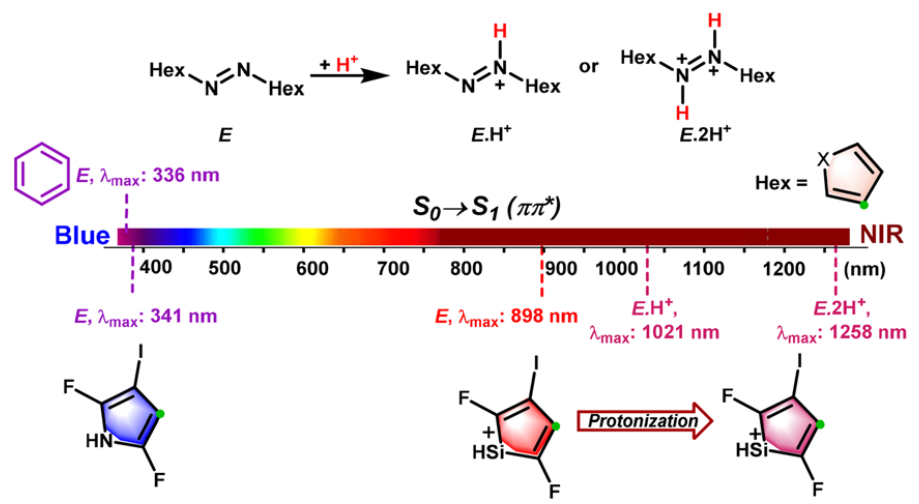
The photoisomerism of azo switches using light in the near-IR region (NIR, 780-1400 nm) is highly preferable for the applications to biomedical and pharmacological fields. The common chemical modifications of azobenzene only enables the E ⇆ Z photoswitching wavelengths of azobenzene derivatives close to the red limit of near-infrared light.
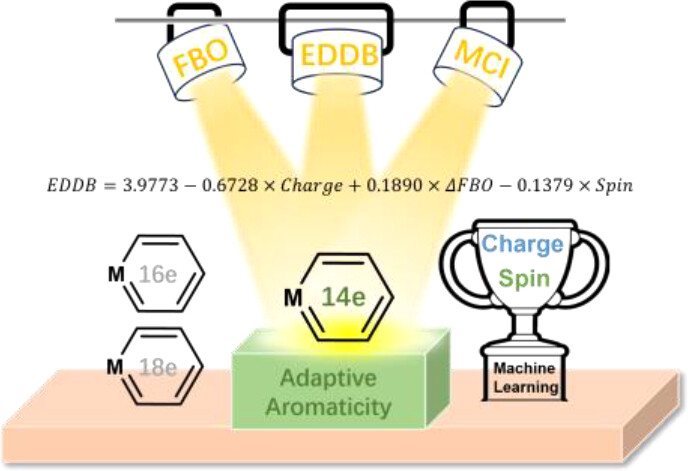
In accordance with the constraints by Hückel’s and Baird’s rules, species generally exhibit aromaticity in one state (the lowest singlet state S0 or the lowest triplet state T1). Consequently, species with adaptive aromaticity (being aromatic in both the S0 and T1 states) are particularly rare. In this study, density functional theory (DFT) was employed to investigate adaptive aromaticity in 14e–, 16e– and 18e– metallabenzenes.
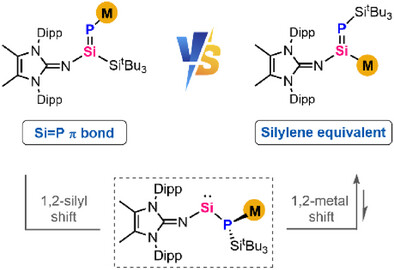
Anionic reagents with silicon-containing double bonds, M(R)Si═ERn (E = main group elements), have garnered significant interest owing to their unique metal-mediated reactivity and their potential in transferring the Si═E unit. Within this domain, the intriguing field of Si-metalated phosphasilenes remains uncharted.
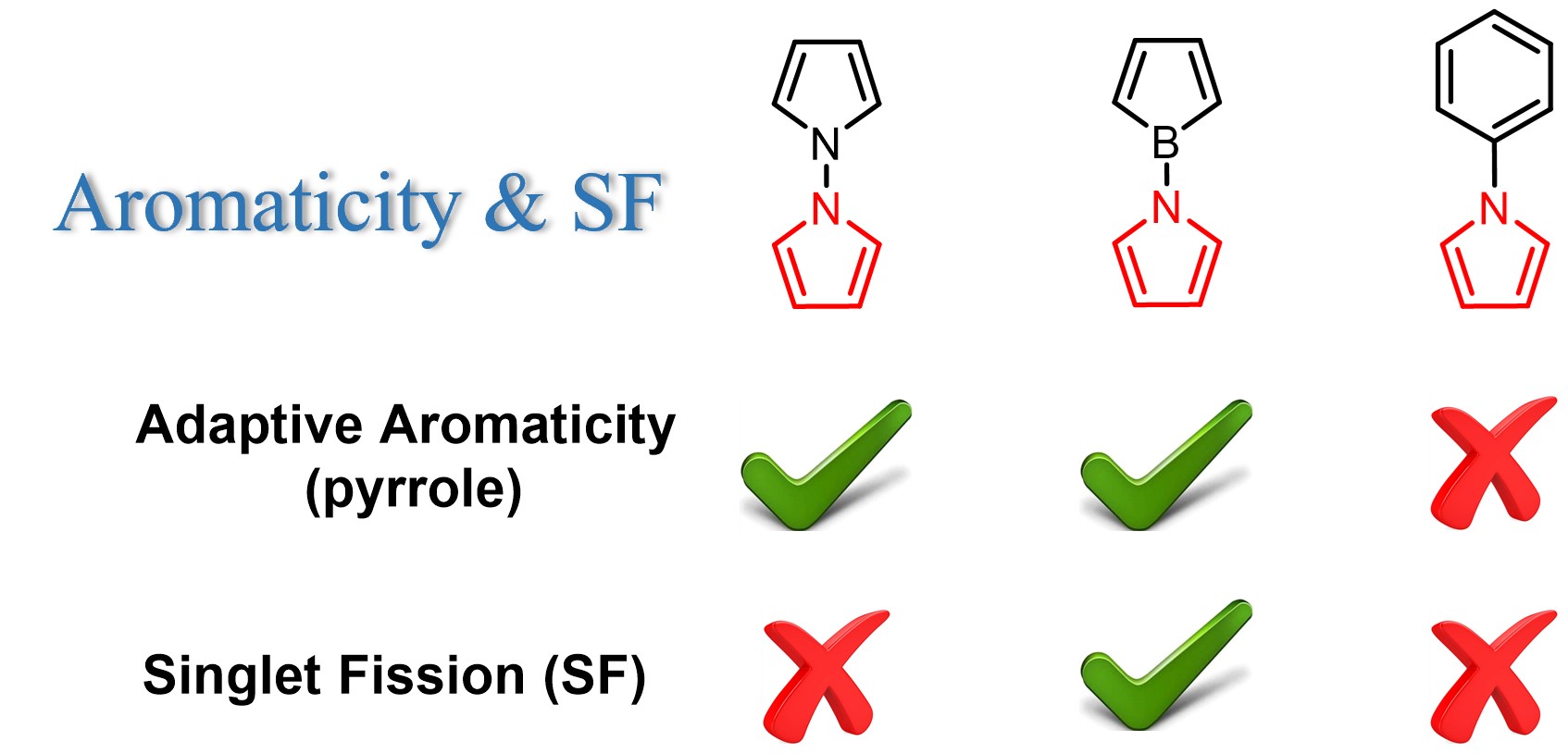
Adaptive aromaticity has attracted substantial attention due to its unconventional two-state aromaticity in both the lowest singlet state (S0) and the lowest triplet state (T1) and its application to the singlet fission. Here we carry out a comprehensive investigation on a series of pyrrole derivatives by density functional theory (DFT) calculations and machine learning.
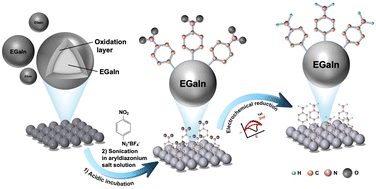
Surface modification of Eutectic Gallium Indium (EGaIn) to inhibit oxidation has been a long-term challenge in materials science, with limited research reporting the formation of a covalent bond between Ga and modifiers for stability purposes. Taking advantage of the strong reductive properties of EGaIn, this study developed a simple method for spontaneous reduction of aryldiazonium salts on the EGaIn nanoparticle surface to form stable covalent Ga–C sigma bonds, effectively suppressing surface oxidation.
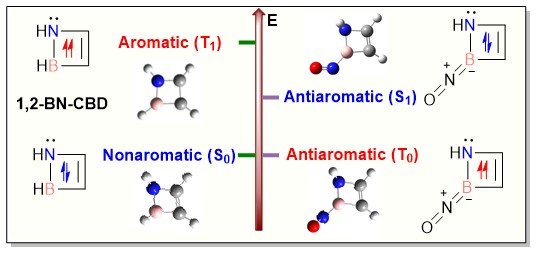
Controlling aromaticity across electronic states is crucial for designing novel species. While aromaticity typically could be achieved in either the lowest singlet state (S0) or the lowest triplet state (T₁), dual-state aromaticity or antiaromaticity remains less developed. Herein, we demonstrate that NO-substitution uniquely induces antiaromaticity in both S0 and T1 states of 1,2-BN-doped cyclobutadiene (1,2-BN-CBD), initially nonaromatic in S0 and weakly aromatic in T1.
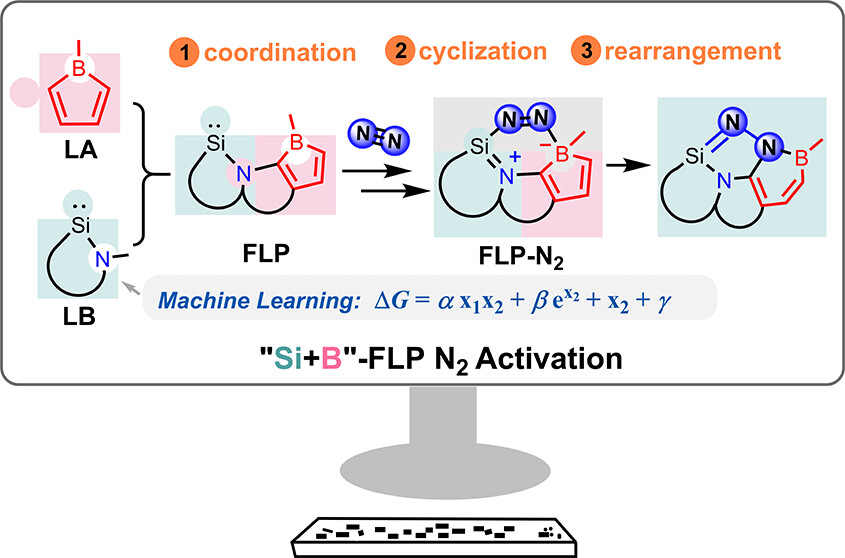
In recent years, while main-group elements from the s- and p-block have emerged in the field of N2 activation, silylenes─despite their remarkable successes in the activation of diverse small molecules─remain unreported for N2 activation. Herein, we design “silylene-borole” frustrated Lewis pairs (FLPs) by combining silylene moieties with boron components and conduct comprehensive density functional theory (DFT) calculations to thoroughly investigate their potential for N2 activation.
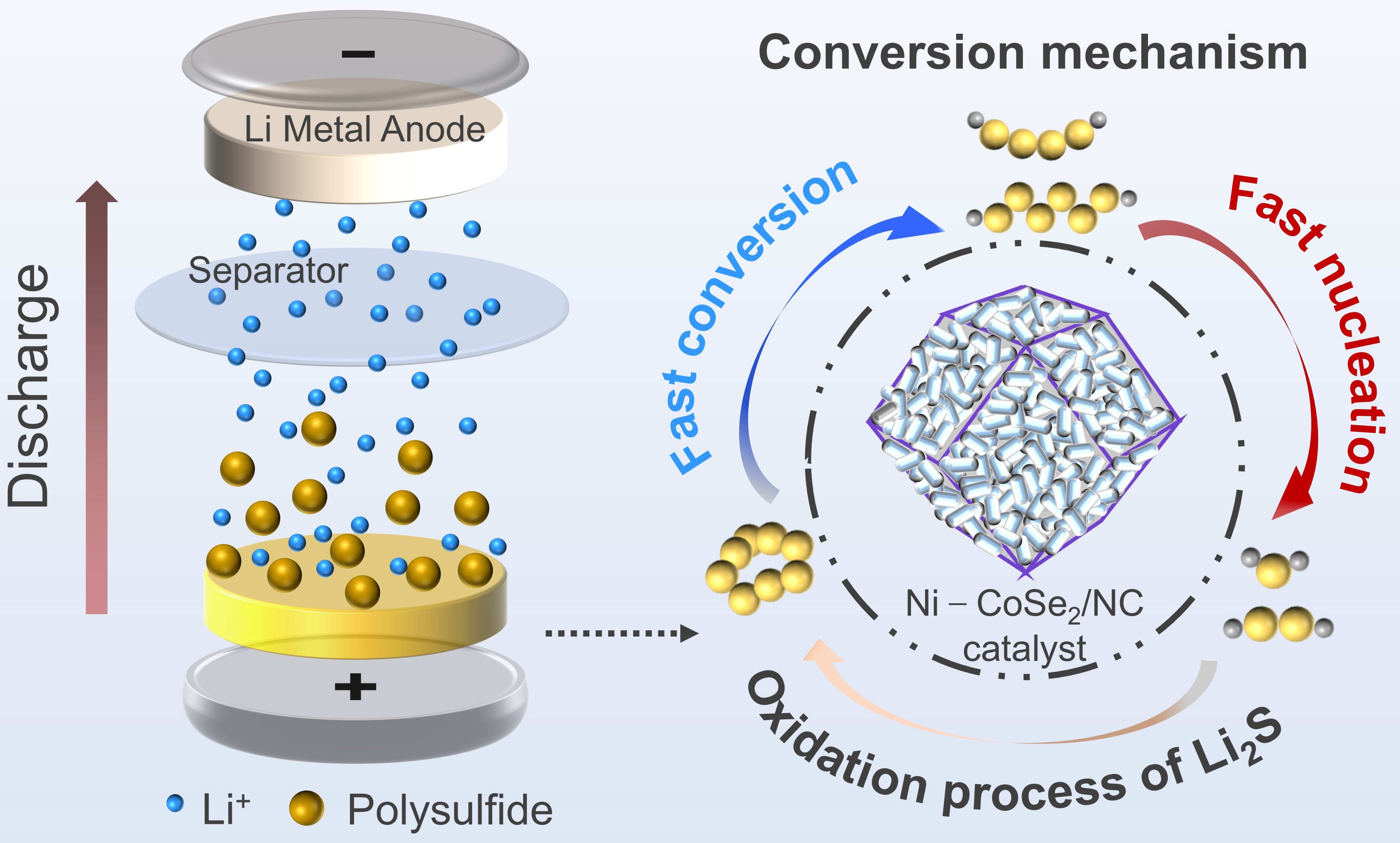
Lithium-sulfur (Li‒S) batteries are regarded as highly promising next-generation energy storage technologies due to their high theoretical specific energy (2600 Wh kg–1), low cost, and the abundance of sulfur. However, their practical application is severely hindered by the shuttle effect of soluble lithium polysulfides (LiPSs) and sluggish sulfur redox kinetics, leading to rapid capacity degradation. The inherent electronic structure of CoSe2, employed as a catalyst, restricts its catalytic efficiency.
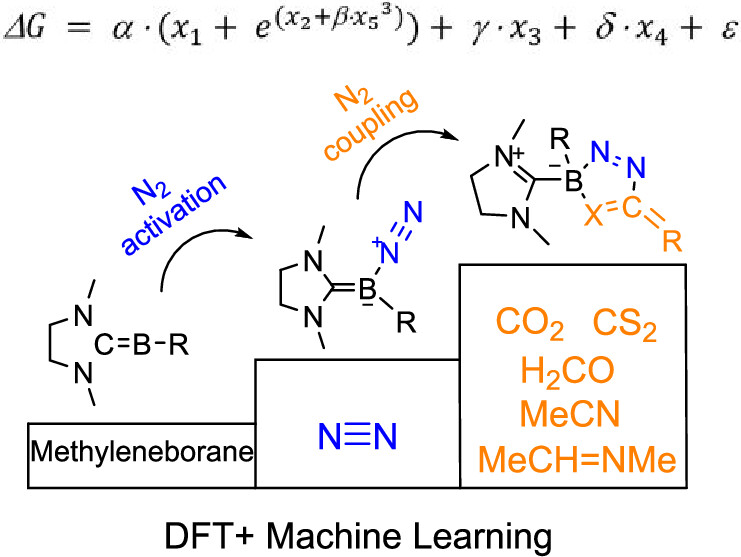
The capture of carbon dioxide is extremely important due to the increasingly severe greenhouse effect, and the conversion of dinitrogen into high-value N–C compounds is of great significance. Here, we predict through density functional theory calculations that the coupling of dinitrogen with carbon dioxide by methyleneborane becomes favorable both thermodynamically and kinetically. Machine learning analysis suggests that increasing the HOMO–LUMO gap or the charge on the boron atom or decreasing the charge of the nitrogen atom will reduce the reaction energies.
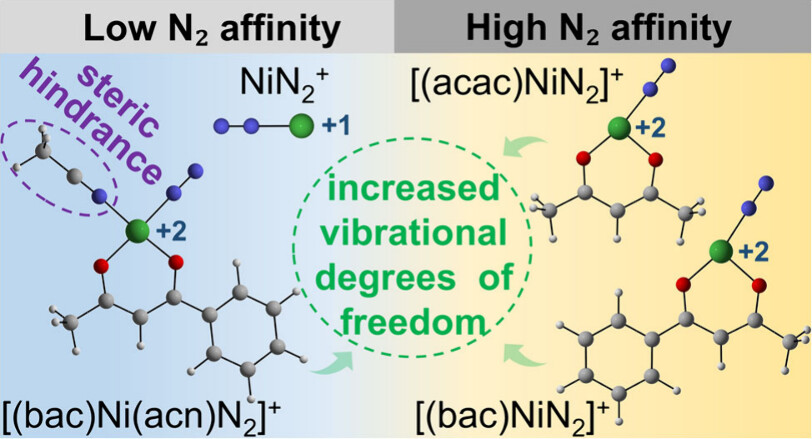
N2 adsorption on metal sites under mild conditions and its dependence on the chemical environment are crucial for understanding the fundamental mechanism and designing an efficient catalyst. Here, electrospray ionization mass spectrometry (ESI-MS) experiments reveal distinct differences in N2 binding capabilities among nickel complexes: both [(acetylacetonato)Ni]+ and [(benzoylacetonato)Ni]+ gas-phase cations exhibit high N2 adsorption efficiency, while the bare Ni+ and [(benzoylacetone)Ni(acetonitrile)]+ cations show negligible reactivity toward N2.
Copyright © 2026,
Theme Originally Created by Devsaran
