Nature Chemistry


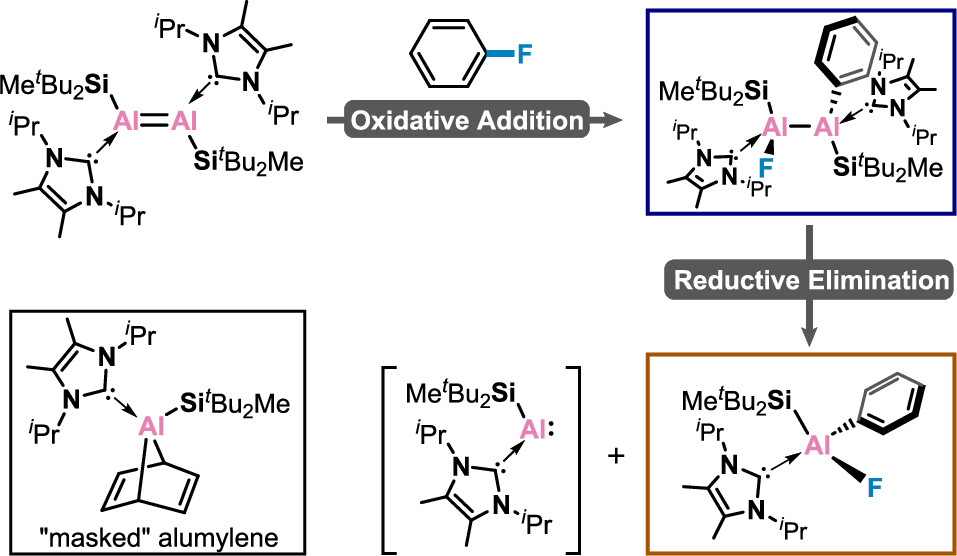
The activation of C–F bonds has long been regarded as the subject of research in organometallic chemistry, given their synthetic relevance and the fact that fluorine is the most abundant halogen in the Earth’s crust. However, C–F bond activation remains a largely unsolved challenge due to the high bond dissociation energies, which was historically dominated by transition metal complexes. Main group elements that can cleave unactivated monofluorobenzene are still quite rare and restricted to s-block complexes with a biphilic nature.
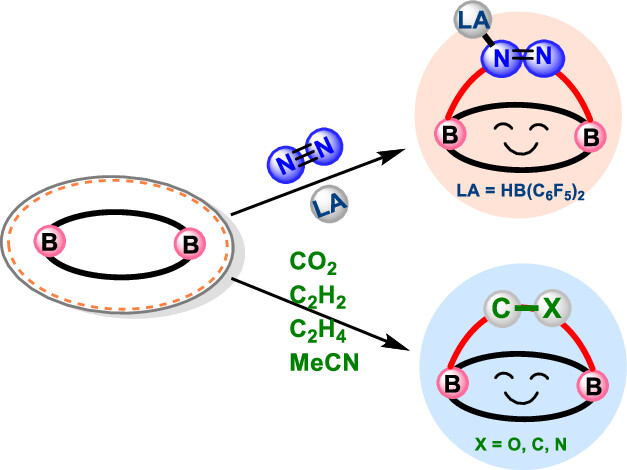
Although main group species have emerged in the field of dinitrogen activation in recent years, the reported examples are particularly rare in comparison with transition metal complexes due to their significant challenges. Herein, we demonstrate a [4 + 2] cycloaddition reaction of N2 (with an activation energy as low as 12.5 kcal mol–1) initiated by an inorganic benzene via density functional theory calculations. Such N2 activation is supported by the elongated nitrogen–nitrogen bond distance (dNN), decreased vibration frequency (νNN), and weakened Wiberg bond index (WBINN).
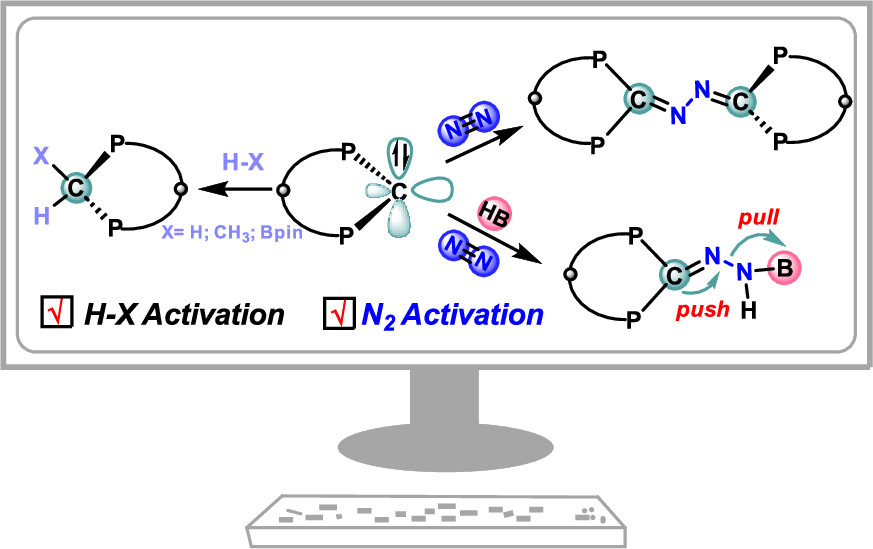
Although the main group species in the s and p blocks have begun to gain prominence in the field of dinitrogen (N2) activation in recent years, reports of carbene-mediated N2 activation remain particularly rare, especially for carbenes with a σ0π2 electronic configuration. Herein, we demonstrate examples of N2 activation initiated by a carbene with a σ0π2 electronic configuration and consequent N2 hydroboration reaction (with a reaction barrier as low as 19.9 kcal/mol) via density functional theory calculations.
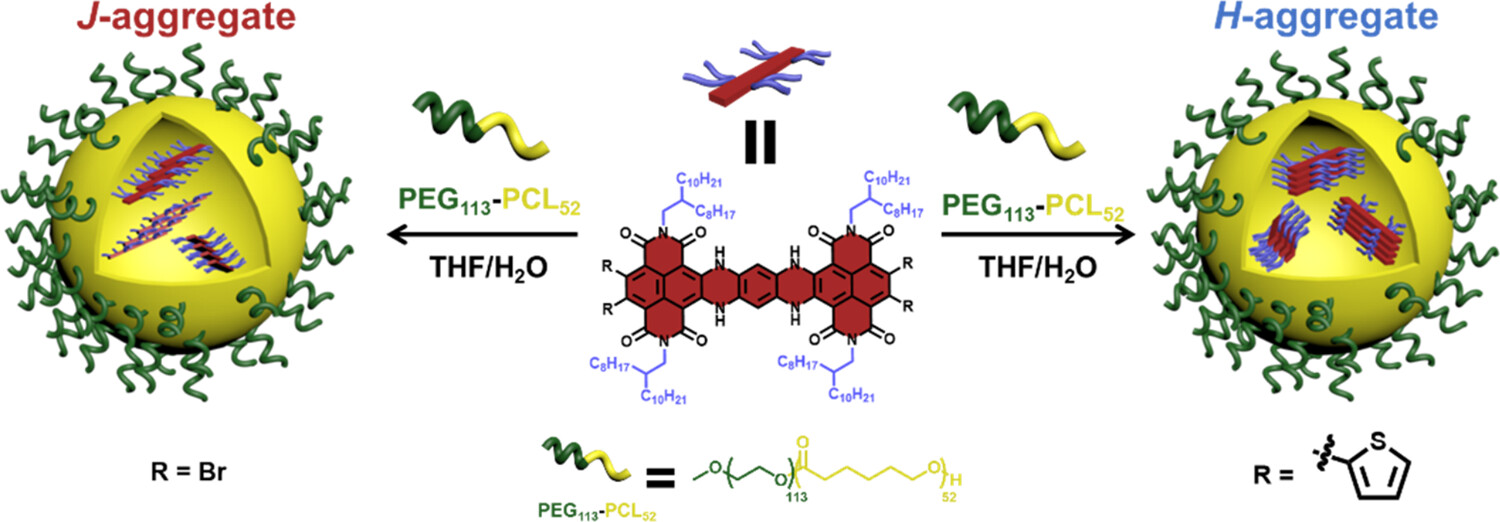
Rational control of the supramolecular aggregation of π-conjugated molecules plays an important role in determining their optoelectronic properties and applications. Here, we report a systematic study of the factors, including solvent polarity, concentration, and surfactants, that affect the aggregation behavior of a brominated hydroazaheptacene tetraimide (HATI) and its thiophene-substituted derivative, Th-HATI, as near-infrared fluorophores, in both nonpolar and polar solvents.
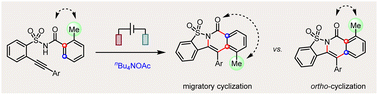
Efficient control over several possible reaction pathways of free radicals is the chemical basis of their highly selective transformations. Among various competing reaction pathways, sulfonimidyl radicals generated from the electrolysis of 2-alkynylbenzenesulfonamides undergo cascade migratory or ortho-cyclization cyclization selectively.

The activation of dinitrogen is significant as nitrogen-containing compounds play an important role in industries. However, the inert NN triple bond caused by its large HOMO-LUMO gap (10.8 eV) and high bond dissociation energy (945 kJ mol−1) renders its activation under mild conditions particularly challenging. Recent progress shows that a few main group species can mimic transition metal complexes to activate dinitrogen. Here, we demonstrate that a series of seven-electron (7e) boron-centered radical can be used to activate N2 via density functional theory calculations.

Tuning the spin state of metal carbynes, which have broad applications in organic synthesis and material science, presents a formidable challenge for modern chemists as the strong field nature of carbyne ligands dictates low-spin ground spin states (S = 0 or 1/2) for known metal carbynes. Through the oxidative addition reaction of a low-coordinate iron(0) N-heterocyclic carbene complex with the C−S bond of a thioazole-2-ylidene, we synthesized the first triplet (S = 1) metal terminal carbyne, an iron cyclic carbyne complex.
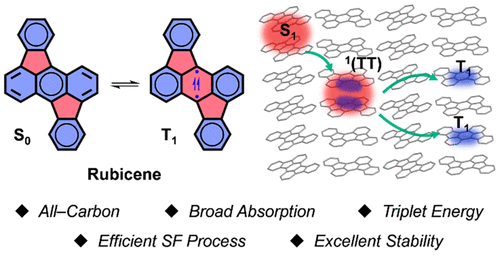
Singlet fission (SF) processes hold great potential in boosting conversion efficiency of solar cells. However, practical applications were greatly hindered by the limited availability of suitable SF materials. Current studies mainly focus on acene derivatives, which are known to be subjected to knotty stability issues or low energy levels. Therefore, developing efficient and stable SF materials is a primary issue before the implementation of practical application. Herein, we present a new SF material based on a rubicene (Rc) skeleton as a desirable acene alternative.

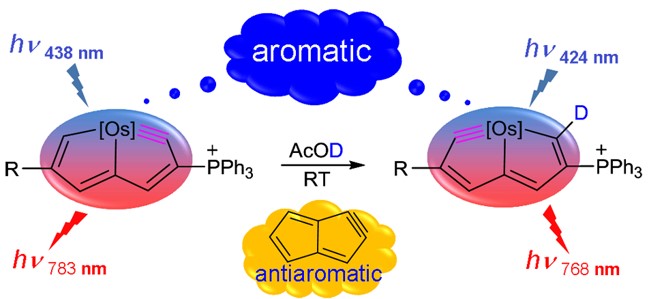
The classification of π-/σ-aromaticity depends on the electrons with the dominating contributions. Traditionally, π- and σ-aromaticity are used to describe the unsaturated and saturated systems, respectively. Thus, it is rarely reported that π-aromaticity is dominated in a saturated system. Here we demonstrate that π-aromaticity could be dominating in several fully saturated four-membered rings (4MRs), supported by various aromaticity indices including ΔBL, NICS, EDDB, MCI, and AdNDP.

Cyclobutadiene (CBD) displays aromaticity in the lowest-lying triplet excited state (T1) according to Baird's 4n electron rule. Hence, antiaromatic CBD in the T1 state has never been reported so far. Here we demonstrate via density functional theory (DFT) calculations that the CBD ring could possess dual antiaromaticity in the lowest singlet state (S0) and T1 states (termed as adaptive antiaromaticity), which is supported by various aromaticity indices including NICS, ACID, ΔBL, ELF and ISE.
Copyright © 2026,
Theme Originally Created by Devsaran
