Nature Chemistry


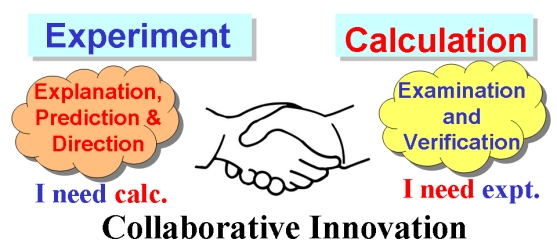

Nitroso compounds, R-N═O, containing N═O double bonds are ubiquitous and widely utilized in organic synthesis. In contrast, heavier congeners of nitroso compounds, namely pnictinidene chalcogenides R-Pn = E (Pn = P, As, Sb, Bi; E = O, S, Se, Te), are highly reactive and scarce. They have been stabilized in the coordination sphere of Lewis acid/base or by pronounced contribution from resonance structures, whereas free species with unperturbed pnictogen-chalcogen double bonds remains elusive.
The concept of adaptive aromaticity, which denotes two-state aromaticity in both the lowest singlet and triplet states, stands in marked contrast to the traditional one-state aromaticity governed by the Hückel and Baird rules. Nonetheless, organic compounds exhibiting adaptive aromaticity remain particularly rare.
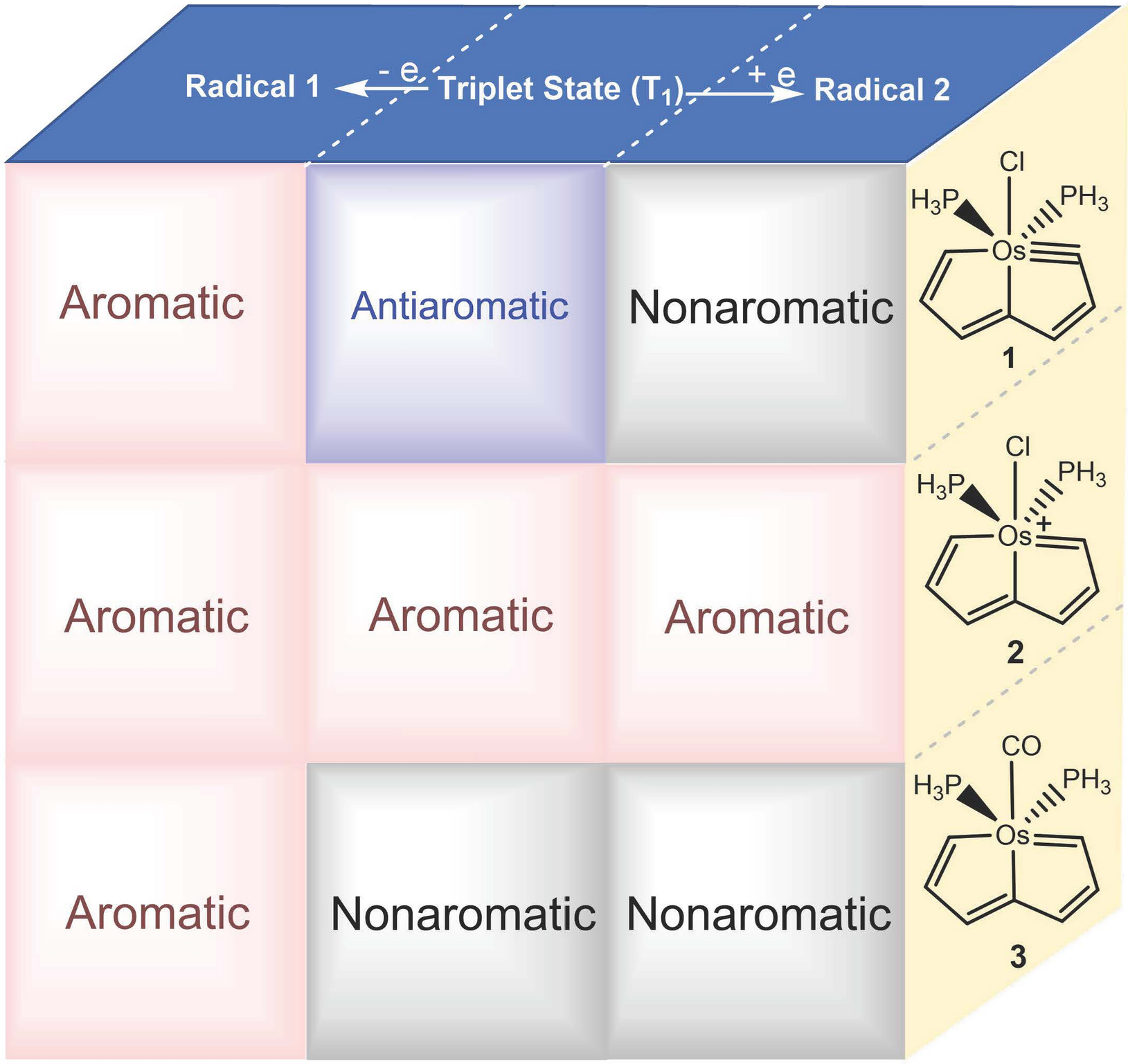
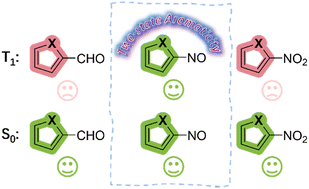
As one of the most important concepts in organic chemistry, aromaticity has attracted considerable attention from both theoretical and experimental chemists. Limited by the traditional rules (Hückel’s rules and Baird’s rules), species can only achieve aromaticity in a single state (S0 or T1) in most cases. In 2018, our group first reported 16 electron osmapentalene that showed aromaticity in both the S0 and T1 states, which is defined as adaptive aromaticity.
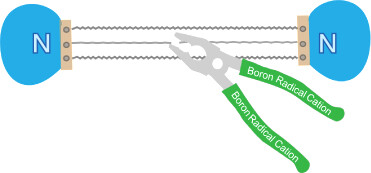
Activation of dinitrogen (N2) under mild conditions has been a particularly challenging project for decades, owing to the highly strong N≡N triple bond. In recent years, the main group species have emerged as a prominent strategy in the field of dinitrogen activation, but the reported examples remain particularly rare compared with transition metal complexes. Herein, we performed a comprehensive density functional theory (DFT) calculation of N2 activation by boron radical cations.
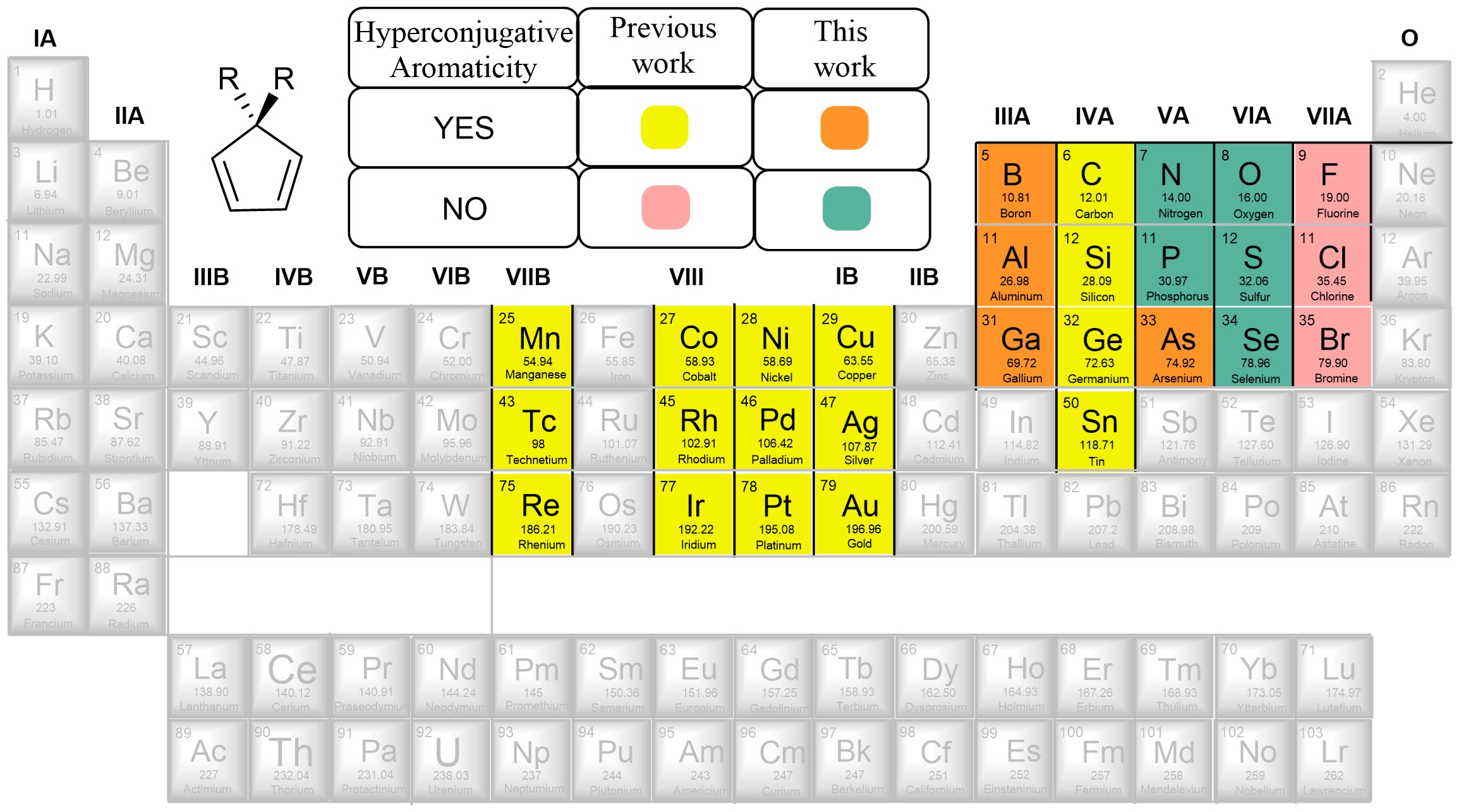
Combining aromaticity and hyperconjugation, two important concepts in organic chemistry, leads to hyperconjugative aromaticity, which was first proposed by Mulliken in 1939. However, previous studies on hyperconjugative aromaticity have mainly focused on substituents containing either main-group elements (group 14) or transition metals in groups 7, 9, 10, and 11. In this study, we perform density functional theory (DFT) calculations on cyclopentadiene and pyrrolium derivatives containing groups 13, 15 and 16 substituents to examine the possibility of achieving hyperconjugative aromaticity.
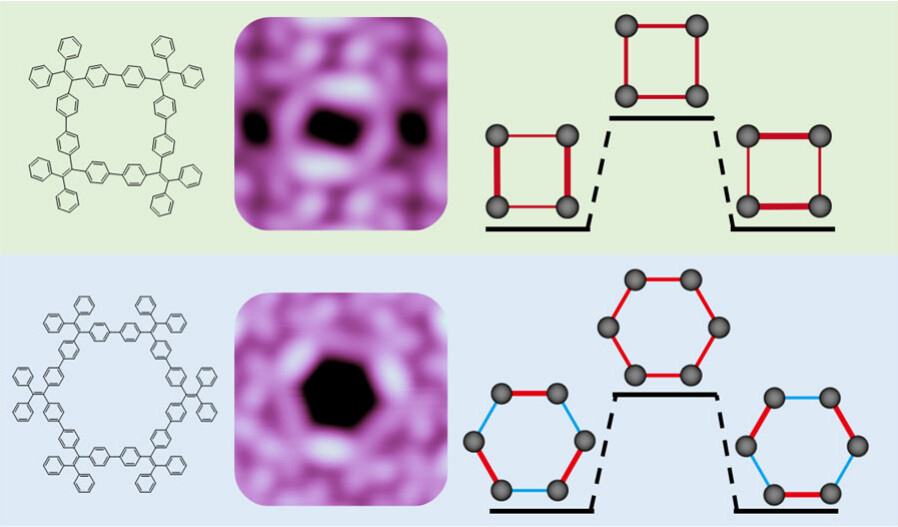
Tetraphenylethylene (TPE) is a prototype aggregate-induced emission molecule. TPE-based conjugated macrocycles exhibit unique optical properties due to their peculiar cyclic topology. Because the symmetry of macrocycles strongly affects their photophysical properties, here we report a single-molecule study of the structures and orbitals of two TPE-based macrocycles of (C26H18)4 and (C26H18)6. Using scanning tunneling microscopy and spectroscopy, we discover that both macrocycles undergo spontaneous symmetry breaking in their conformations and frontier orbitals.
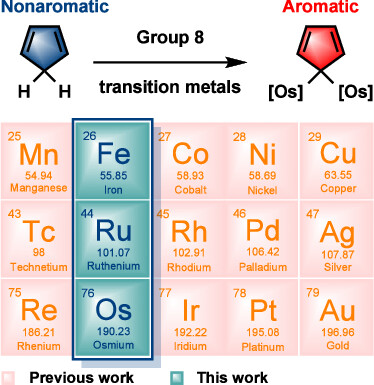
Hyperconjugative aromaticity, an integration of two chemical concepts, aromaticity and hyperconjugation, was first proposed in 1939. Recently, breaking through the main group elements, the hyperconjugative aromaticity has been successfully extended to the transition metal system, including groups 7, 9, 10, and 11 organometallic substituents. Here, we demonstrate that the missing group 8 transition metal substituents also possess a powerful ability to induce hyperconjugative aromaticity in cyclopentadiene via density functional theory calculations.
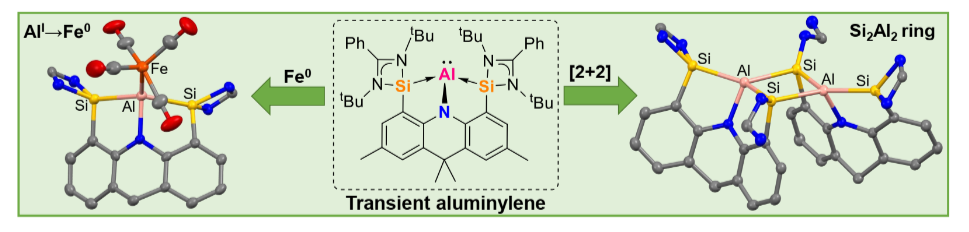
The suitability of electron-rich bis-silylenes, specifically the neutral chelating [SiII(Xant)SiII] ligand (SiII = PhC(NtBu)2Si, Xant = 9,9dimethylxanthene) and the anionic [SiII(NAcrid)SiII)]‒ pincer ligand (NAcrid = 2,7,9,9-tetramethylacridane), has been successfully probed to stabilize monovalent bis-silylene-supported aluminium complexes (aluminylenes).
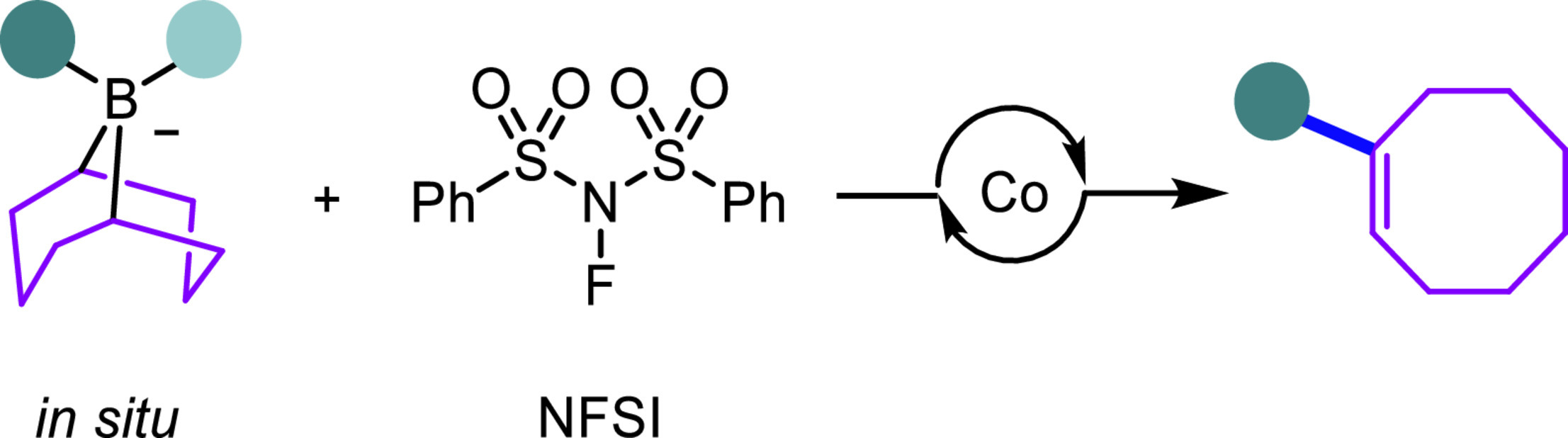
In most Suzuki-Miyaura carbon-carbon cross-coupling reactions, the borabicyclo[3.3.1]nonane scaffold (9-BBN) only serves as an auxiliary facilitating the transmetalation step and thus is transformed into by-products. There are rare examples where the 9-BBN derivatives serve as the potentially diverse C8 building blocks in cross-coupling reactions. Herein, we report a cobalt-catalyzed migratory carbon-carbon cross-coupling reaction of the in situ formed 9-BBN ate complexes to afford diverse aryl- and alkyl-functionalized cyclooctenes.
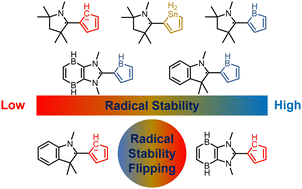
Aromaticity, as a classical and fundamental concept in chemistry, can enhance thermodynamic stability. In sharp contrast, a previous study showed that antiaromaticity rather than aromaticity can enhance the radical stability of α-methyl heterocyclic compounds. Here, we demonstrate a similar antiaromaticity-promoted radical stability when the methyl group is replaced by five-membered (alkyl)(amino)cyclics (AACs). More interestingly, when an AAC is fused with an antiaromatic ring, the radical stability could be either reduced or enhanced, depending on the spin population.
Copyright © 2026,
Theme Originally Created by Devsaran
