Nature Chemistry



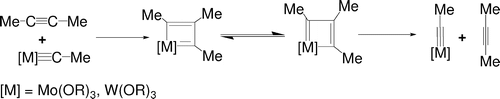
In this paper, the mechanism of alkyne metathesis catalyzed by W/Mo alkylidyne complexes has been theoretically investigated with the aid of density functional theory calculations. Calculations on various model alkylidyne complexes M( CMe)(OR)(3) (M = W, Mo; R = Me, CH2F), W( CMe)(NMe2)(3), and W( CMe)(Cl)(3) allow us to examine the factors that influence the reaction barriers. In the reaction mechanism, metallacyclobutadienes are initially formed from a ring-closing step between alkynes and alkylidyne complexes. A ring-opening step then gives the metathesis products.
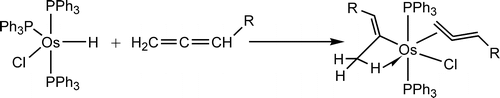
Treatment of OsHCl(PPh3)(3) with allenes CH2=C=CHR at room temperature in benzene produced the vinyl complexes OsCl(C(CH3)=CHR)(CH2=C=CHR)-(PPh3)(2), instead of eta(3)-allyl complexes as normally observed. DFT calculations show that the formation of the vinyl complex is favored kinetically.

Palladium-catalyzed terminal alkyne dimerization, through oxidative homocoupling, is a useful approach to the synthesis of symmetrical 1,4-diynes. Recent investigations have suggested that this reaction might be accomplished in the absence of intentionally added stoichiometric oxidants (to reoxidize Pd(0) to Pd(II)). In this paper, we have fully addressed the question of whether oxygen (or added oxidant) is required to facilitate this process. The presence of a stoichiometric quantity of air (or added oxidant such as I2) is essential for alkyne dimerization.
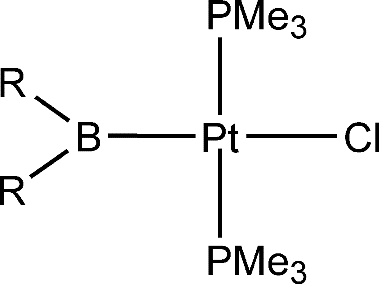
In this paper, the trans influence of boryl ligands, together with that of other ligands commonly believed to have a strong trans influence, has been investigated theoretically via density functional theory (DFT) calculations on a series of square-planar platinum(II) complexes of the form trans-[PtL(Cl) (PMe3)(2)].
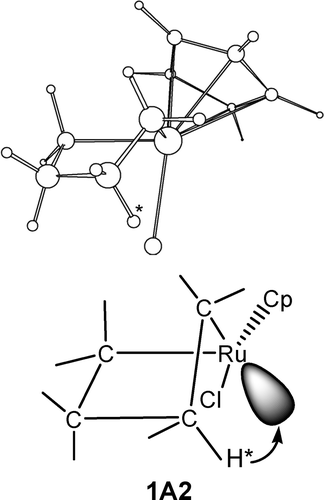
B3LYP density functional theory calculations have been carried out to examine the structural and energetic aspects of β-hydrogen elimination in several metallacyclic complexes of ruthenium and platinum. Factors affecting barriers of the elimination reactions have been examined. It was found that favorable structural arrangements, in which the transferring β-hydrogen is in close proximity to the metal center, for β-hydrogen elimination exist in certain ring conformations of metallacyclic complexes.
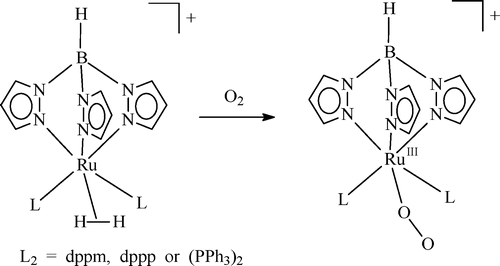
The η2-dihydrogen complex [TpRu(L2)(H2)]+ (L2 = dppm, dppp, or (PPh3)2) prepared in situ by protonation of the hydride precursor reacts with O2 to yield the paramagnetic RuIII-superoxo complex [TpRuIII(L2)(O2)]+, in which antiferromagnetic coupling between the RuIII ion (d5, S = 1/2) and the coordinated superoxide radical (S = 1/2) does not seem to be present.
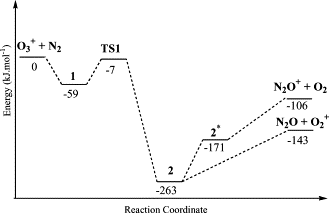
Plausible mechanisms for reactions of the ionized O-3 with N-2 are studied by DFT and electron correlation methods. Calculations show that formation of the primary products O-2(+) + N2O and N2O+ + O-2 arises from an intermediate [O-2...ON2](+) in the ground state and its charge-transfer excited state, respectively. New routes to NO2 + NO+ through an intermediate [ON2...O-2](+) and to [ON...NO](+) + O-2 via the reactions of O-3(+) with N2O are proposed.
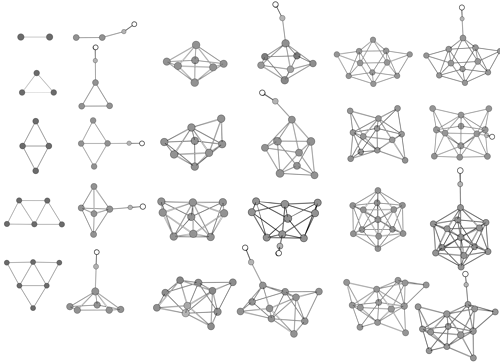
Density functional calculations for copper clusters Cu and their monocarbonyls CunCO (n less than or equal to 13) have been performed using the relativistic ECP plus DZ basis set augmented by an f polarization function for copper atom. Equilibrium geometries, harmonic frequencies, and static mean polarizabilities of Cu-n and CunCO are determined. The feature of CO adsorption on the copper cluster and the effect of CO adsorption on stability and polarizability of the cluster are investigated.

Density functional theory with the B3LYP functional is used to calculate the equilibrium geometries and harmonic vibrational frequencies of nitryl halogenides XNO2 and XONO (X = F, Cl, Br, I). Stabilities and isomerizations of these isomers are investigated. Dissociation energies of the X-N bond in XNO2 are predicted at the B3LYP/6-311G* and QCISD(T)/ce-pvTZ levels. The electronic transition energies of the most stable XNO2 species have been estimated by time-dependent B3LYP calculations.
Copyright © 2026,
Theme Originally Created by Devsaran
