Activation of the S-S bonds of alkyl disulfides RSSR (R = Me, Et, Pr, Bu-n) by heterodinuclear phosphido-bridged CpW(Co)(2)(mu-PPh2)Mo(CO)(5)
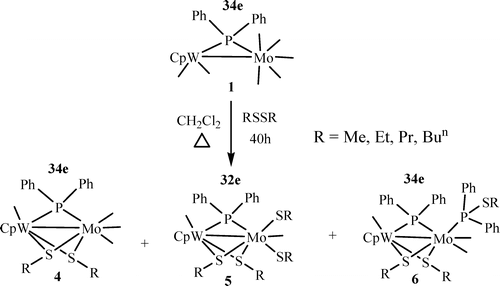
Reactions of CpW(CO)(2)(mu-PPh2)Mo(CO)(5) (1) with alkyl disulfides RSSR (R = Me, Et, Pr, Bu-n) in refluxing dichloromethane yielded the series of new mixed-metal and mixed-ligand bridged compounds CpW(CO)(mu-SR)(2)(mu-PPh2)Mo(CO)(3) (R = Me (4a), Et (4b), Pr (4c), Bun (4d)), CpW(CO)(mu-SR)2(mu-PPh2)Mo(CO)(mu-SR)(2) (R = Me (5a), Et (5b), Pr (5c), Bu-n (5d)), and CpW(CO)(mu-SR)(2)(mu-PPh2) Mo(CO)(2)(PPh2SR) (R = Me (6a), Et (6b), Pr (6c), Bu-n (6d)). All except 6c were characterized by single-crystal X-ray diffraction analysis. Formation of compounds 4-6 indicates a general procedure for cleavage of the S-S bonds of alkyl disulfides under mild conditions. Molecular structures of compounds 6a,b,d reveal the first transformation of the bridging PPh2 ligand of 1 to give the hybrid ligands Ph2PSR (R Me, Et, Bun) via P-S bond formation. The average Mo-W bond distance (2.8255 angstrom) in the 34e dimers (4a-d, 6a,b,d) is shorter than that in the 32e dimers (5a-d), 2.8494 angstrom. This appears quite unusual, according to the 18e rule. DFT calculations have been performed to investigate this unusual observation. Characterization of the substitution products CpW(CO)(mu-SMe)(2)(mu-PPh2)Mo(CO)(2)PPh2Me (7) and CpW(CO)(mu-SMe)(2)(mu-PPh2) Mo(CO)(COD) (8; COD = cyclooctadiene) leads to the conclusion that carbonyl ligands on the Mo sites are more labile than those on the W sites.
