Nature Chemistry



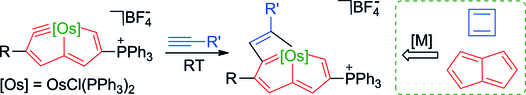
Antiaromatic species are substantially less thermodynamically stable than aromatic moieties. Herein, we report the stabilization of two classical antiaromatic frameworks, cyclobutadiene and pentalene, by introducing one metal fragment through the first [2+2] cycloaddition reaction of a late-transition-metal carbyne with alkynes. Experimental observations and theoretical calculations reveal that the metal fragment decreases the antiaromaticity in cyclobutadiene and pentalene simultaneously, leading to air- and moisture-stable products.
For details, please check the link at http://onlinelibrary.wiley.com/doi/10.1002/anie.201501349/abstract
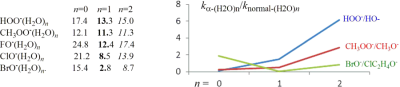
To probe the kinetic performance of microsolvated α-nucleophile, the G2(+)M calculations were carried out for the gas-phase SN2 reactions of monohydrated and dihydrated α-oxy-nucleophiles XO−(H2O)n = 1,2 (X = HO, CH3O, F, Cl, Br), and α-sulfur-nucleophile, HSS−(H2O)n = 1,2, toward CH3Cl. We compared the reactivities of hydrated α-nucleophiles to those of hydrated normal nucleophiles.
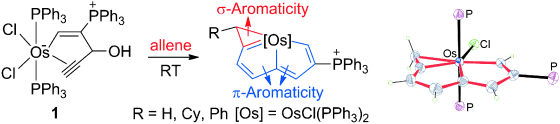
In general, aromaticity can be clarified as π- and σ-aromaticity according to the type of electrons with major contributions. The traditional π-aromaticity generally describes the π-conjugation in fully unsaturated rings whereas σ-aromaticity may stabilize fully saturated rings with delocalization caused by σ-electron conjugation. Reported herein is an example of σ-aromaticity in an unsaturated three-membered ring (3 MR), which is supported by experimental observations and theoretical calculations.
For details, please check the link at http://onlinelibrary.wiley.com/doi/10.1002/anie.201411220/abstract
Please note that it is the calculations that discover the σ-Aromaticity in such a uniqu system.
For details, please check the link at http://onlinelibrary.wiley.com/doi/10.1002/asia.201590003/abstract
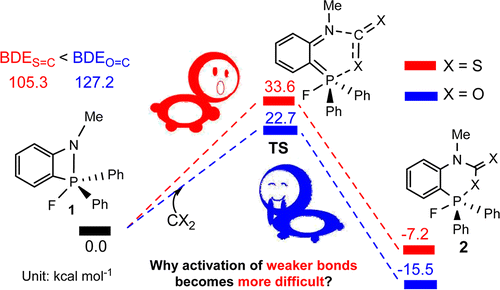
The sequestration of carbon disulfide (CS2), a common pollutant in environmental systems, is of great importance due to its physical harm to human beings. Compared with CO2 capture, that of CS2 is much less developed. The use of P/N-based frustrated Lewis pairs (FLPs) has been proven, both experimentally and theoretically, to be an alternative strategy to efficiently sequestrate CO2. Therefore, we pose the question of whether the analogue CS2 could also be sequestrated by the same FLPs, given that the C═S bond in CS2 is weaker than the C═O bond in CO2.
Dr. Zhu introduced the group projects to Prof. Pierre H. Dixneuf in his office on Dec 1st, 2014.
Congratulations!
Ms. Xuerui Wang is awarded the National Graduate Studentship. Congratulations!
Copyright © 2026,
Theme Originally Created by Devsaran
