Nature Chemistry



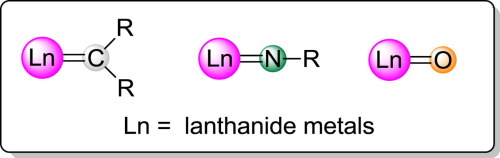
Metal-ligand multiple bonds have received significant attention in the past few decades. A series of novel species with lanthanide-ligand multiple bonds have recently been isolated. This short review summarizes the synthesis and reactivity of these novel complexes.
https://www.sciencedirect.com/science/article/pii/S0040403917316234

Aromaticity, one of the central topics in chemistry, has attracted continuing interest of both experimentalists and theoreticians.

Although the formation of metal–carbon σ bonds is a fundamental principle in organometallic chemistry, the direct bonding of one organic molecule with one metal center to generate more than two metal–carbon σ bonds remains a challenge. Herein, we report an aromaticity-driven method whereby multiyne chains are used to construct three metal–carbon σ bonds in a one-pot reaction under mild conditions. In this method, multiyne chains act as ligand precursors capable of chelating an osmium center to yield planar metallapolycycles, which exhibit aromaticity and good stability.
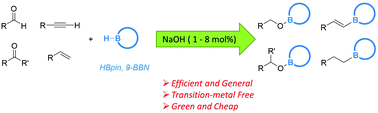
Commercially available NaOH powder is shown to be an efficient transition-metal-free initiator for the catalytic hydroboration of aldehydes, ketones, alkynes and alkenes with HBpin and 9-BBN under mild conditions. Combined experimental and theoretical studies suggest that the catalytically active species is a boron hydride generated in situ from the reaction mixture.
http://pubs.rsc.org/en/content/articlelanding/2017/gc/c7gc01632h#!divAbstract
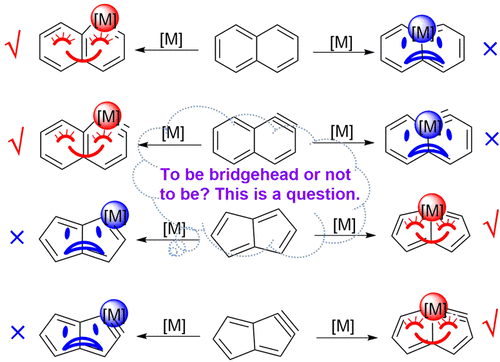
Transition-metal-containing metallaaromatics have attracted considerable interest from both experimental and computational chemists because they can display properties of both organometallic compounds and aromatic organic compounds. In general, the transition metal in a metallabicycle prefers a nonbridged position to the bridgehead one because of the larger ring strain caused by the rigidity in the bridgehead position, as exemplified by metallanaphthalene and metallanaphthalyne.
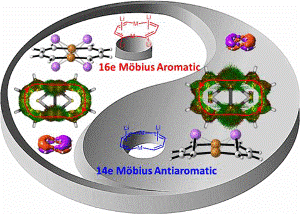
Aromaticity, one of the most fundamental concepts in chemistry, can be classified as Hückel- and Möbius-type according to the electron count and topology. In comparison with numerous Hückel aromatics containing 4n+2 π-electrons, Möbius aromatics with 4n π-electrons, especially the Craig-type species are particularly limited.
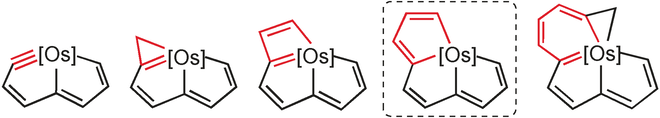
Polycyclic complexes containing a bridgehead transition metal are interesting species because the transition metal is shared by all the rings simultaneously. In this study, we present a novel osmium–bridgehead system with three fused five-membered rings. This novel framework can be viewed as a 10-atom carbon chain coordinating to the osmium center. In sharp contrast to the nonplanar organic analogue, this unique metallacycle exhibits good planarity, which was unambiguously verified by means of X-ray diffraction.
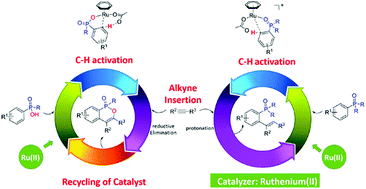
Using density functional theory (DFT) calculations, the present study explores the mechanisms of two ruthenium(II)-catalyzed phosphoryl-directed ortho-selective C–H bond activation reactions. Depending on the nature of the phosphoryl groups, namely R2P(O) versus RP(O)OH, two different products could be selectively synthesized. For R2P(O), the overall catalytic cycle includes three basic steps: C–H bond activation, alkyne insertion, and protonation. The oxidation state of the Ru center does not change during this catalytic process.
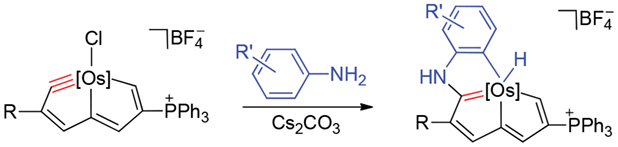
Treatment of osmapentalyne [Os{≡C-C(COOMe)=CH-C=CH-C(PPh3)=CH-}Cl(PPh3)2]+BF4- with arylamines in the presence of Cs2CO3 produced osmium-bridged polycyclic aromatic complexes. In this reaction, metal carbyne of osmapentalyne was first attacked by nucleophiles, followed by a C-H oxidative addition. The UV-Vis spectra of these osmium-bridged polycyclic aromatic complexes were measured. The result shows that these osmium-bridged polycyclic aromatic complexes have broad absorption in the UV-Vis region up to 650 nm.
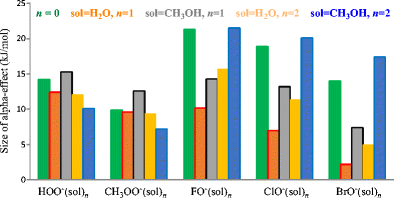
The modified G4(MP2) method was applied to explore microsolvation effects on the reactivity of four solvated normal oxy-nucleophiles YO−(CH3OH)n=1,2 (Y = CH3, C2H5, FC2H4, ClC2H4), and five α-oxy-nucleophiles YO−(CH3OH)n=1,2 (Y = HO, CH3O, F, Cl, Br), in gas-phase SN2 reactions towards the substrate CH3Cl.
Copyright © 2026,
Theme Originally Created by Devsaran
