Nature Chemistry



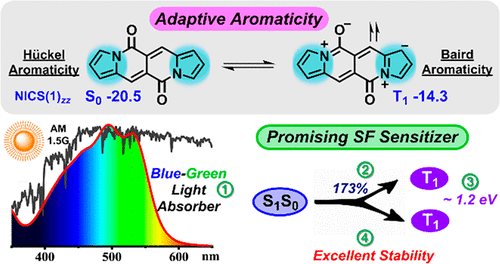
Singlet fission (SF) materials hold the potential to increase the power conversion efficiency of solar cells by reducing the thermalization of high-energy excited states. The major hurdle in realizing this potential is the limited scope of SF-active materials with high fission efficiency, suitable energy levels, and sufficient chemical stability.
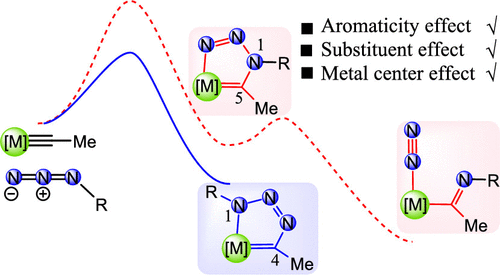
Density functional theory calculations were used to investigate the reaction mechanisms on [3 + 2] cycloaddition reactions of azides with metal carbyne complexes. Our results reveal that the formation of a 1,4-metallatriazole regioisomer is a kinetically favorable process in comparison with the formation of 1,5-metallatriazole. Aromaticity plays an important role in stabilizing the products in these reactions. Further analyses show that the electron-donating ligand on metal centers or the electron-withdrawing group on the azide could accelerate the [3 + 2] cycloaddition reaction.
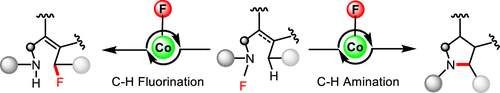
A redox neutral radical-relay cobalt-catalyzed intramolecular C–H fluorination of N-fluoroamides featuring the in situ formed cobalt fluorides as the latent radical fluorinating agents is reported. Moreover, the reactivity of such a cobalt catalysis could be diverted from C–H fluorination to amination by engineering substrates’ conformational flexibility. Preliminary mechanistic studies (UV–vis spectroscopy, cyclic voltammetry studies and DFT calculations, etc.) support the reaction proceeding a redox neutral radical-relay mechanism.

The synthesis of conjugated Möbius molecules remains elusive since twisted and macrocyclic structures are low entropy species sporting their own synthetic challenges. Here we report the synthesis of a Möbius macrocycle in 84% yield from the alkyne metathesis of 2,13-bispropynyl[5]helicene. MALDI-MS, NMR, and X-ray diffraction indicated a trimeric product of two-fold symmetry with PPM/MMP configurations in the helicene subunits.

Adaptive aromaticity in the lowest singlet and triplet states is a rare property found among molecular systems. So far, only osmapentalene and osmapyridinium have been found to possess the adaptive aromaticity. Although it has been confirmed that the pattern of electron excitation is a key factor to achieve the adaptive aromaticity, further investigation of the metal center effect has not yet been made. Ruthenium, another Group 8 transition metal, can form metallacycles similar to the osmium counterparts.

Aromaticity is one of the most basic concepts in organic chemistry. The planar Möbius aromatic metallapentalynes and metallapentalenes have been attracted considerable attention in the past few years. However, the aromaticity of metallapentalenes containing heteroatoms (such as B, N, and O), termed as hetero‐metallapentalenes, is rarely studied. Here, we theoretically investigated the stability and aromaticity of a series of hetero‐metallapentalenes.

The never-ending pursuits for exploring aromatic molecular architectures result in the large libraries of aromatics with fascinating structures, which have greatly broadened the scope of aromaticity. Despite extensive efforts that have been paid to develop aromatic frameworks, the construction of polycyclic aromatics that share a bridgehead atom with more than three rings has never been accomplished.

Molecular nitrogen (N2), an abundant component of the atmosphere, is appealing for industrial value‐added products. However, its intrinsic inertness limits its activation to mainly metallic species. Environmental concerns and harsh reaction conditions have resulted in a demand for alternate nonmetallic and nontoxic routes to activate and functionalize N2 at ambient conditions. Comprehensive density functional theory (DFT) calculations are performed on N2 activation by boron species, specifically for the experimentally more accessible tricoordinated boron compounds.
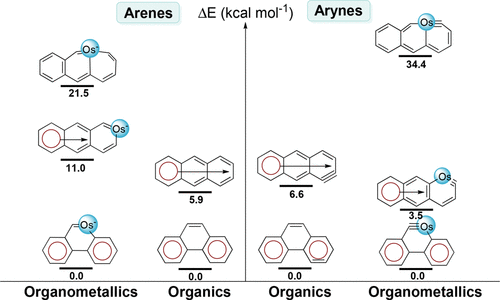
Metallaaromatics have attracted considerable attention in recent years because they can display properties of both organic and organometallic species. However, it remains unclear whether Clar’s rule could be applied to organometallic chemistry despite its proposal in 1950s. Here, we investigate the relative stabilities of 49 organic and organometallic species by density functional theory (DFT) calculations.
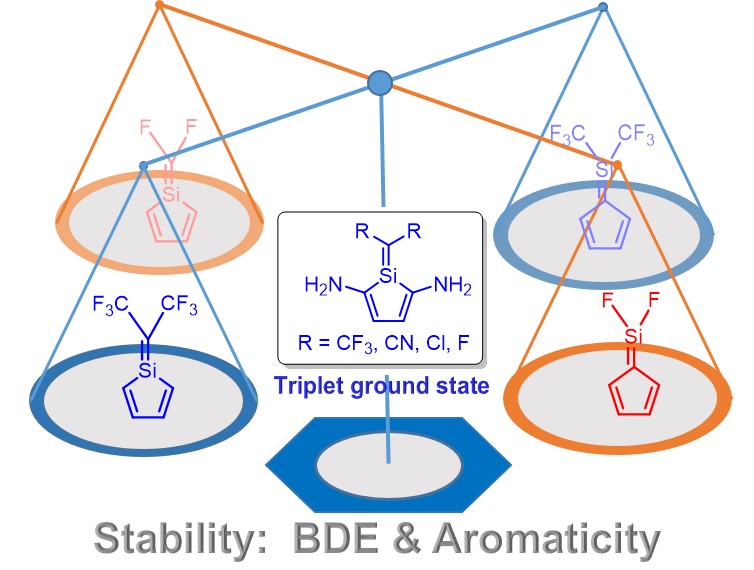
Pentafulvenes are dipolar hydrocarbons since they shift their π-electrons to achieve Hückel aromaticity and thus the electron donating groups at the exocyclic position can enhance their aromaticity. Silapentafulvenes are analogues of pentafulvene formed by the replacement of the carbon atoms at the exocyclic CC double bond with a silicon atom in pentafulvene. It remains unclear how the aromaticity of 5-silapentafulvenes and 6-silapentafulvenes can be changed due to the polarization of the CSi double bond.
Copyright © 2026,
Theme Originally Created by Devsaran
