Nature Chemistry




The σ-bond activation by main group element has received enormous attention from theoretical and experimental chemists. Here, the reaction of C-X (X = Cl, Br, I) bonds in benzyl and allyl halides with a pincer-type phosphorus(III) species was reported. A series of structurally robust phosphorus(V) compounds were formed via the formal oxidative addition reactions of C-X bonds to the phosphorus(III) center. Density functional theory calculations show that the nucleophilic addition process is more favorable than the direct oxidative addition mechanism.

The isolated‐pentagon rule (IPR) is a determining structural feature accounting for hollow fullerene stabilization and properties related to Cn (n ≥ 60) cages. The recent characterization of an unprecedented non‐IPR hydrofullerene, C2v‐C66H4, bearing two heptagons with adjacent fused‐pentagons motif, largely dismiss this feature. Herein, employing DFT calculations, we explore the 13C‐NMR pattern and aromatic behavior of C2v‐C66H4.

Activation of dinitrogen (N2, 78%) and dioxygen (O2, 21%) has fascinated chemists and biochemists for decades. The industrial conversion of N2 to ammonia requires extremely high temperatures and pressure. Here we report the first example of N2 and O2 cleavage by a uranium complex, [N(CH2CH2NPiPr2)3U]2(TMEDA), under ambient conditions without an external reducing agent. The N2 triple bond breaking implies a U(III)-P(III) six-electron reduction. The hydrolysis of the N2 reduction product allows the formation of ammonia or nitrogen-containing organic compound.
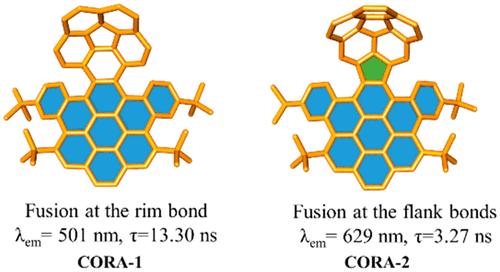
The selective fusions with pyrene derivative to the rim and flank bonds of corannulene generated 4 and 7, respectively, which underwent a Scholl reaction to provide novel distorted PAHs CORA-1 and CORA-2, consisting of corannulene and dibenzocoronene units with different connections between them. The studies revealed that the properties of these PAHs are highly dependent on the fusing positions of corannulene.

The Baird’s rule has been applied to a large scope of organic molecular systems for rationalizing the aromaticity reversal in the lowest-lying triplet state. In this study, we demonstrate that the Baird’s rule can be also extended to all-metal systems with σ- and π-aromaticity.
https://pubs.rsc.org/en/Content/ArticleLanding/2020/CC/D0CC05586G#!divAbstract
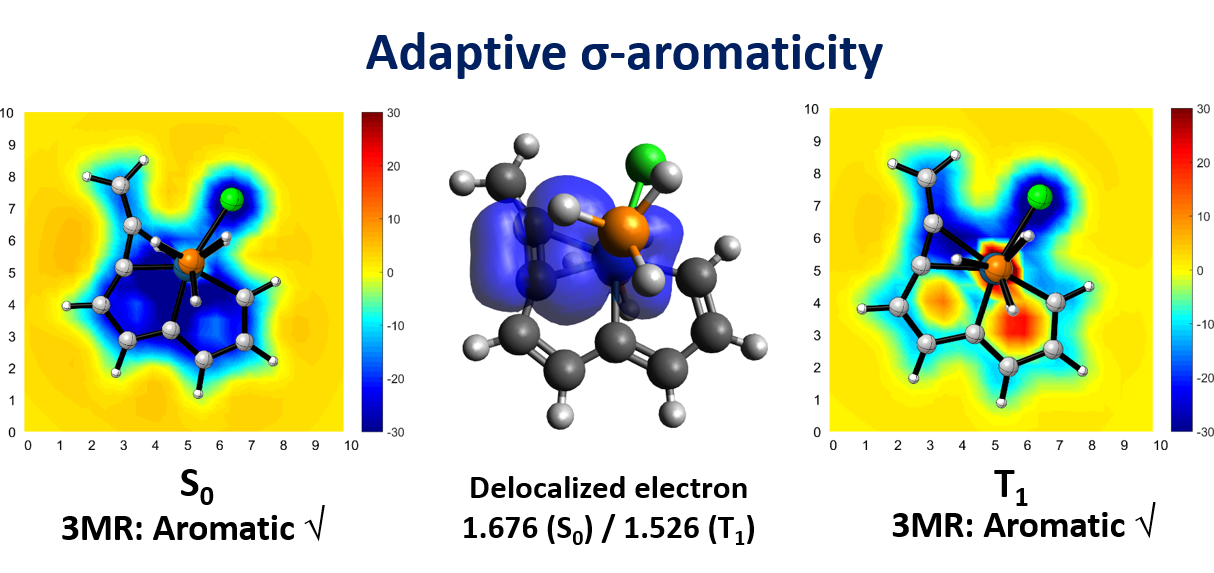
Based on Hückel’s and Baird’s rules, species are aromatic either in the lowest singlet state (S0) or the lowest triplet state (T1) only. Thus, species with adaptive aromaticity (with aromaticity in both the S0 and T1 states) is particularly rare. On the other hand, σ-aromaticity in the T1 state has been underdeveloped, let alone adaptive σ-aromaticity. Herein, via various aromaticity indices including NICS, ACID and EDDB, we demonstrate adaptive s-aromaticity in an unsaturated three-membered ring, which is a traditional area dominated by π-aromaticity.
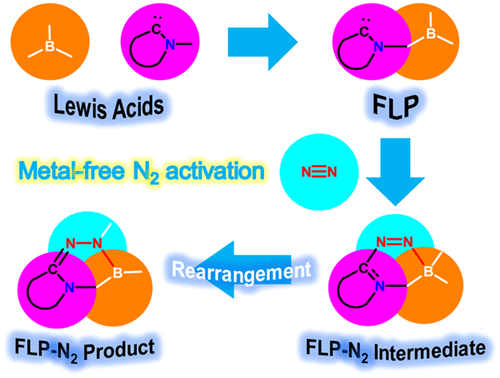
Activation of the strongest triplet bond in molecular nitrogen (N2) under mild conditions is particularly challenging. Recently, its fixation and reduction were achieved by highly reactive dicoordinated borylene species at ambient conditions, ripping the limits of harsh reaction conditions by metallic species. Less reactive species with a facile preparation could be desirable for next-generation N2 activation. Now density functional theory calculations reveal that tricoordinated boranes could be a potential candidate of N2 activation/functionalization.
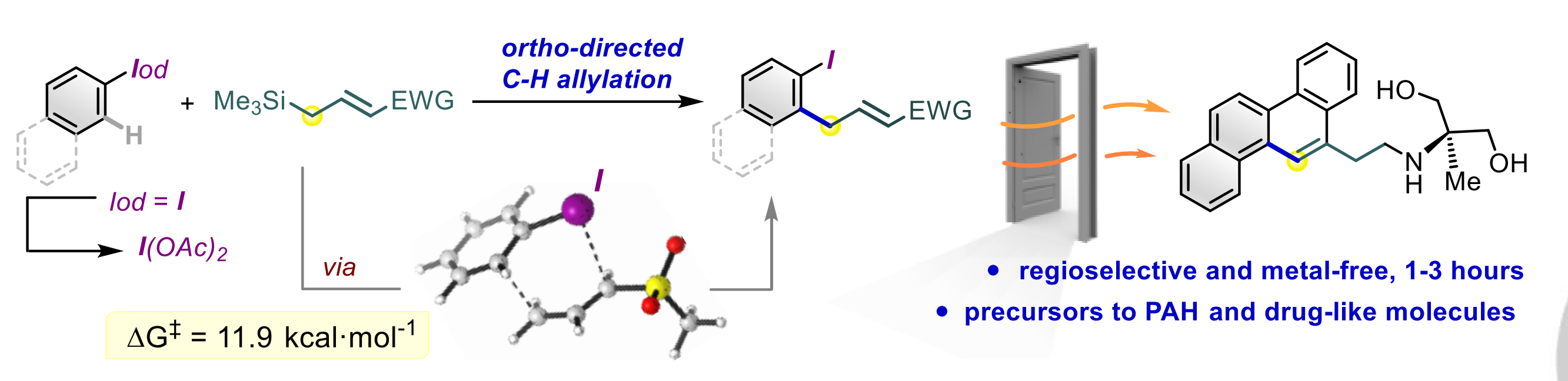
A metal‐free C‐H allylation strategy is described to access diverse functionalized ortho ‐allyl‐iodoarenes. The method employs hypervalent (diacetoxy)iodoarenes and proceeds through the iodane‐guided “iodonio‐Claisen” allyl transfer. The use of allylsilanes bearing electron‐withdrawing functional groups unlocks the functionalization of a broad range of substrates, including electron‐neutral and electron‐poor rings.
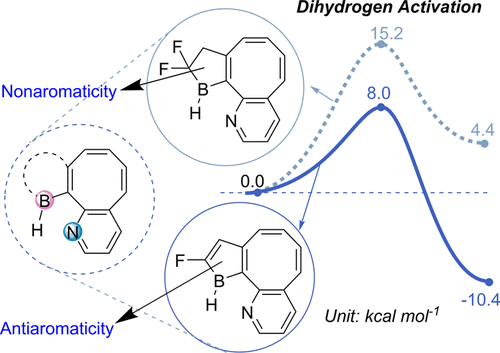
Aromaticity and frustrated Lewis pairs (FLP), two important concepts in chemistry, have attracted considerable attention from theoretical and experimental chemists. However, combining these two concepts together for H2 activation is less developed. Herein, we report a density functional theory study on antiaromaticity-promoted H2 splitting. The antiaromatic borole (as Lewis acid) and aromatic pyridine (as Lewis base) were introduced into the cyclooctetraene skeleton. Due to the geometric constraints, such systems can be classified as FLPs.
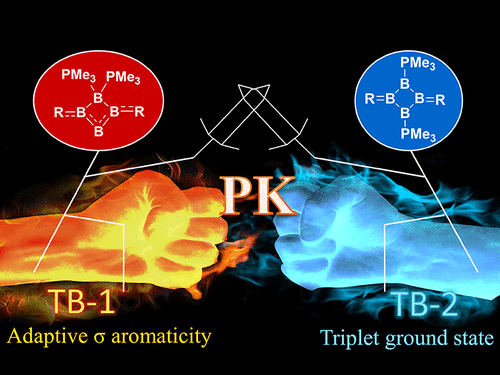
In comparison with the widely recognized π aromaticity, σ aromaticity is a less developed concept in chemistry, especially for unsaturated systems. Moreover, most studies on σ aromaticity have been mainly limited to the ground state of saturated systems; unsaturated species with σ aromaticity in the excited state have never been reported.
Copyright © 2026,
Theme Originally Created by Devsaran
