Nature Chemistry


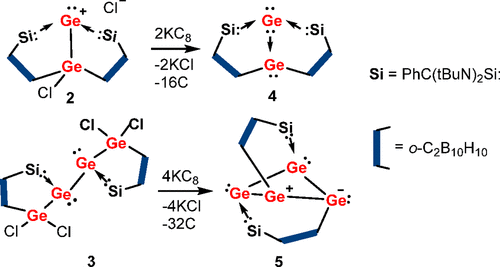
The first Ge(0)–Ge(II) germylone–germylene-paired Ge2 complex (LSi)2Ge2 (4) and the molecular Ge4 cluster (LSi)2Ge4 (5) supported by the chelating carbanionic ortho-C,C′-dicarborandiyl-silylene ligand LSi [L = C,C′-C2B10H10, Si = PhC(tBuN)2Si] have been synthesized and isolated via reduction of the corresponding precursors chlorogermyl-germyliumylidene chloride (2), [(LSi)2Ge(Cl)Ge]+Cl–, and (LSi
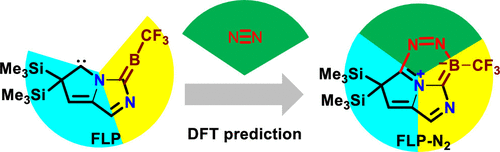
Activation of atmospherically abundant dinitrogen (N2) by metal-free species under mild reaction conditions has been one of the most challenging areas in chemistry for decades. Very recent but limited progress in N2 activation by boron species, including two-coordinated borylene and methyleneborane and three-coordinated borole and borane, has been made toward metal-free N2 activation.
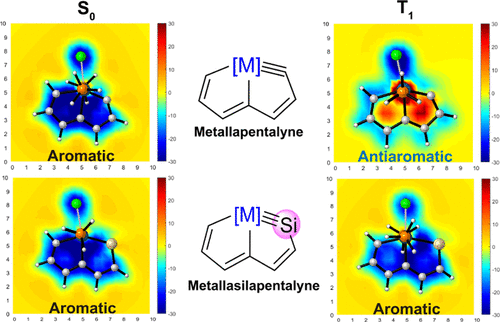
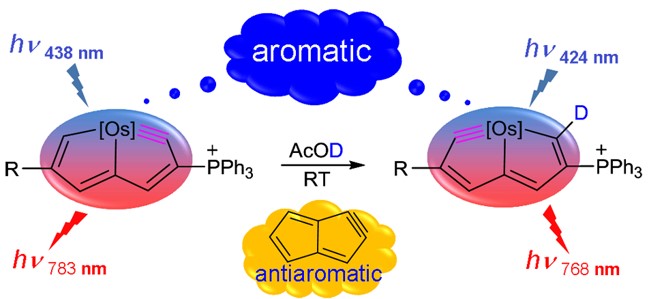
Cyclic molecules with 4n + 2 or 4n electrons are aromatic in the lowest singlet state (S0) or the lowest triplet state (T1) according to Hückel and Baird’s rules. Thus, the design of aromatic species in both the S0 and T1 states (termed as adaptive aromaticity) is particularly challenging. In this work, we demonstrate that metallasilapentalynes show adaptive aromaticity supported by structural, magnetic, and electronic indices, in sharp contrast to metallapentalynes, which exhibit aromaticity in the S0 state only.
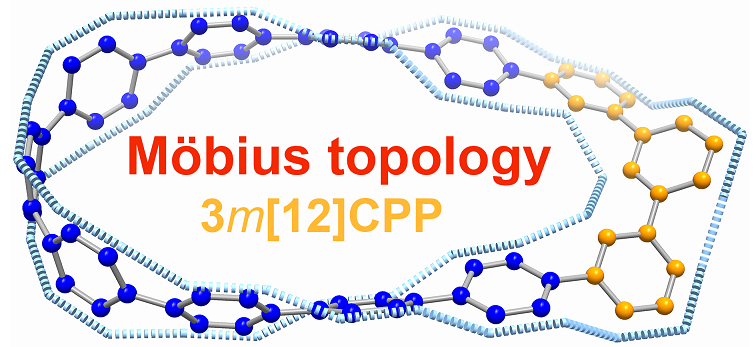
Carbon nanohoop, a class of constrained molecular architecture consisting of linked arene units, has attracted considerable interest from both experimental and theoretical chemists due to their synthetic challenge and aesthetic architectures. Another fascinating and synthetically challenging species, the Möbius-type molecule, has been attracting the scientific community with its elegant structure and aromaticity. Thus, combining two things together, synthesizing a carbon nanohoop with Möbius topology remains more challenging to date.
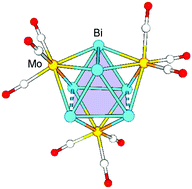
The first Zintl cluster containing a distorted Bi6 triangular prism, [Bi6Mo3(CO)9]4−, has been synthesized and structurally characterized. Quantum chemical calculations indicated that the distorted cage cluster features multiple local σ-aromaticity.
https://pubs.rsc.org/en/content/articlelanding/2021/CC/D1CC00734C#!divAbstract
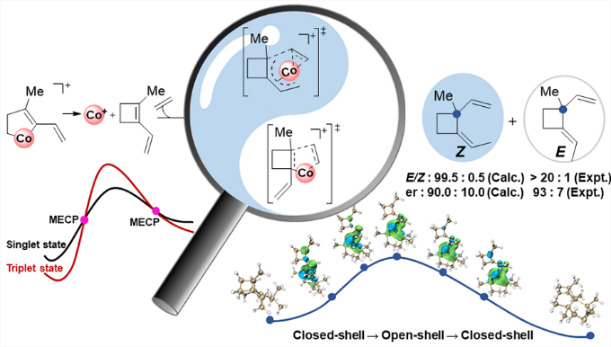
The cyclobutane unit is important to prepare complex natural products with biological activity due to the high ring strain. Among various approaches, [2 + 2] cycloaddition is one of the major strategies to prepare cyclobutane under light conditions. Recently, Rajanbabu's group reported tandem catalysis for asymmetric coupling of inactivated ethylene and enynes to functionalized cyclobutenes or cyclobutanes. However, the reaction mechanisms remain unproven.

Aromaticity and hyperconjugation are two fundamental concepts in organic chemistry. By combination of the two concepts together, the resulting hyperconjugative aromaticity has attracted considerable attention from both theoretical and computational chemists. However, previous studies are mainly focused on the main group chemistry. For the hyperconjugative aromaticity in the transition metal chemistry, the studies are limited to groups 10 and 11.

The Ni‐B complex 1BCF with a facilely accessible monophosphine (PtBu3) unit was theoretically designed, which was found to be more active than that with an ambiphilic ligand for hydrogenation of styrene. Substituting PtBu3 with a stronger electron donating ligand N‐heterocyclic carbene largely improves the activity of the Ni‐B complex.

Disilene has attracted considerable interests due to the trans-bending geometry which is significantly different from the planar alkene. However, the equilibrium between disilene and isomeric silylsilylene has not been fully understood. Here, we report a density functional theory (DFT) study on this equilibrium. Calculations reveal significant effects of substituent, aromaticity and base. Specifically, the parent disilene is thermodynamically more stable than the isomeric silylene.
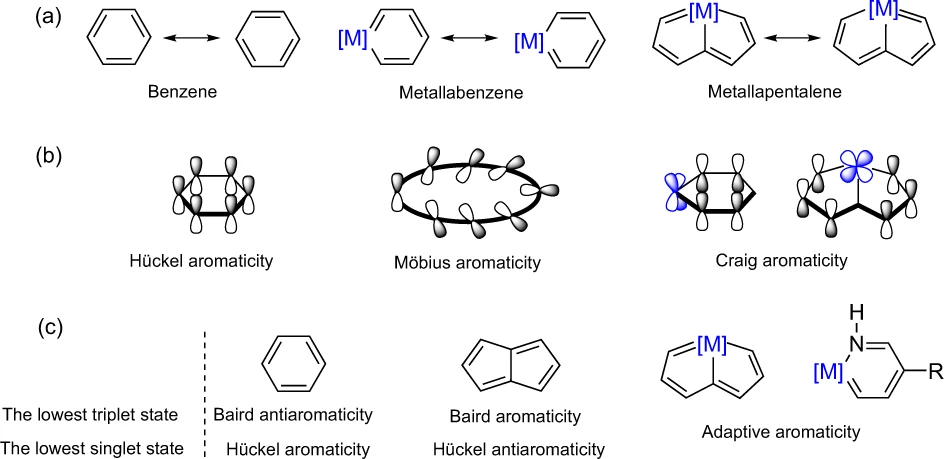
While sp2-hybridized carbon atoms in hydrocarbons typically contribute only one electron to their aromaticity, metals have more electrons from d or f orbitals available for participating in conjugation in organometallics, complicating the electron counting as well as analysis of their aromaticity. Here, the author comments on the challenges towards understanding aromaticity in organometallics and outlines several remaining questions that have yet to be answered.
Copyright © 2026,
Theme Originally Created by Devsaran
