Antiaromaticity-Promoted Activation of Dihydrogen with Borole Fused Cyclooctatetraene Frustrated Lewis Pairs: A Density Functional Theory Study
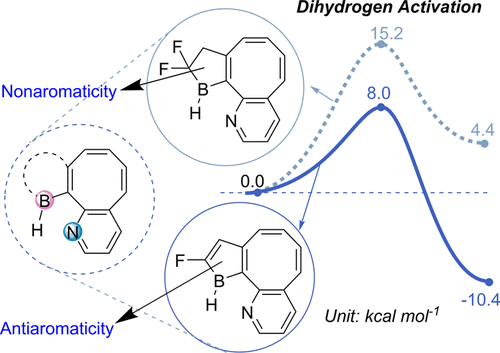
Aromaticity and frustrated Lewis pairs (FLP), two important concepts in chemistry, have attracted considerable attention from theoretical and experimental chemists. However, combining these two concepts together for H2 activation is less developed. Herein, we report a density functional theory study on antiaromaticity-promoted H2 splitting. The antiaromatic borole (as Lewis acid) and aromatic pyridine (as Lewis base) were introduced into the cyclooctetraene skeleton. Due to the geometric constraints, such systems can be classified as FLPs. In the process of hydrogenation, breaking the antiaromatic boron-containing ring can decrease the reaction barrier, whereas breaking an aromatic boron-containing heterocycle makes dihydrogen activation both kinetically and thermodynamically unfavorable. Further investigation reveals that smaller deformation as well as stronger interaction between distorted reactants in those FLPs with antiaromatic rings lead to lower activation barriers in comparison with FLPs with nonaromatic five-membered rings or aromatic six-membered rings. These findings could be helpful to design novel FLPs toward H2 activation.
