Nature Chemistry


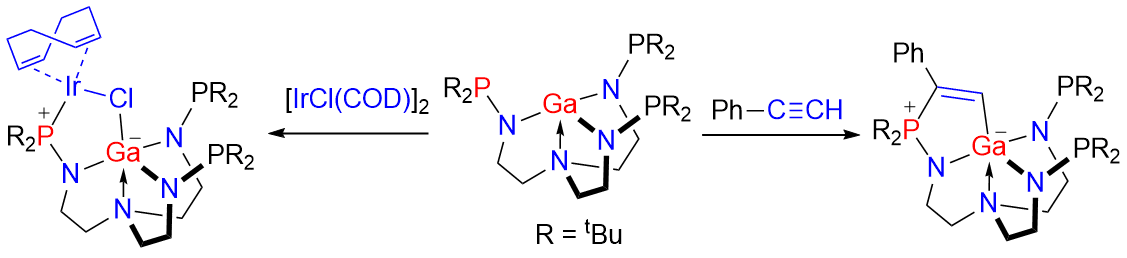
Frustrated Lewis pairs (FLPs) represent a new paradigm of main‐group chemistry. The Lewis acidic centers in FLP chemistry are typically B and Al atoms in the studies reported over the past decade, and most of them are tri‐coordinated with strong electron‐withdrawing groups. Herein, we report a Ga/P system containing an unprecedented four‐coordinated Lewis acidic Ga center. This Ga/P species performs classical addition reactions toward heterocumulenes, alkyne, diazomethane, and transition metal complex. Regioselective formation of the products can be rationalized by DFT calculations.
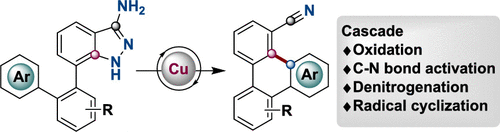
We present a novel Cu-catalyzed aromatic metamorphosis of 3-aminoindazoles via oxidative cleavage of two C–N bonds of 3-aminoindazoles. This unprecedented reactivity of 3-aminoindazoles allows one to forge diverse nitrile-containing triphenylenes in decent yields via generation of the cyano group in situ. The current study reveals that 3-aminoindazoles could be harnessed as radical precursors via oxidative denitrogenation, the reaction mechanism of which was supported by density functional theory calculations.

Unusual 1,2‐migration reactions of N‐heterocyclic carbene (NHC) on transition metals were investigated using density functional theory calculations. Our results reveal that the electronic properties, ring strain of the four‐membered ring, and aromaticity of NHC play crucial roles in the thermodynamics of such a 1,2‐migration.
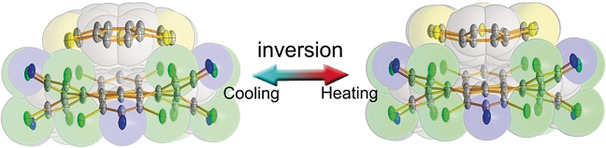
Bowl inversion is a unique property of buckybowls. The polarity and assembly configuration of buckybowls are reversed after bowl inversion. So far, this unique phenomenon has been studied in solution and on surface, but not in solid state due to spatial constraint. Now a series of exo‐type supramolecular assemblies of trithiasumanene and nanographene are investigated. Tuning the electron density of the nanogaphene component was found to directly affect the binding constant of the complex.
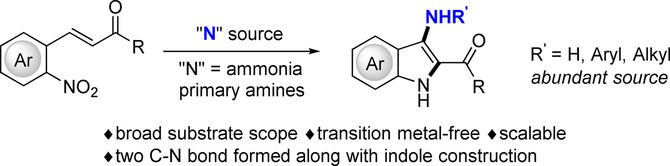
A step‐economic strategy for 3‐aminoindoles synthesis with ammonia or primary amines as “N” source under transition‐metal‐free conditions was achieved. A series of 3‐aminoindoles was obtained with abundant “N” source featuring high efficiency, mild conditions, environmental friendliness and scalability. Efficient syntheses of the intermediates of COX‐2 inhibitor and tubulin polymerization inhibitor were successfully accomplished with this newly developed strategy.

The [1,5]‐migration reaction has attracted considerable attention from experimentalists and theoreticians for decades. Although it has been extensively investigated in various systems, studies on pyrrolium derivatives are underdeveloped. Herein, a theoretical study on the reaction mechanism of [1,5]‐migration in both pyrrolium and pyrrole derivatives is presented.

Fluorine is the most electronegative element in the periodic table. Thus, activation of the carbon–fluorine (C−F) bond, the strongest single bond to carbon, has attracted considerable interest from both experimentalists and theoreticians. In comparison with numerous approaches to activate C−F bonds, the aromaticity‐promoted method is less developed. Herein, we demonstrate that the C−F bond activation could be achieved by a facile tautomerization, benefitting from aromaticity, which can stabilize both the transition states and products.
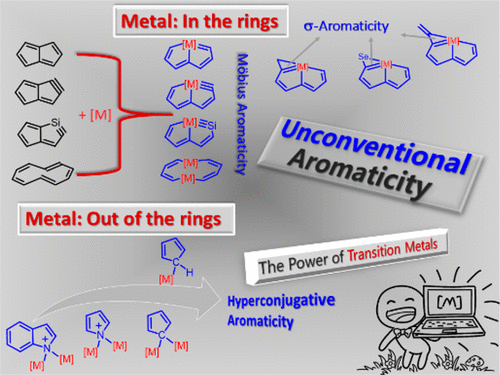
Aromaticity, one of the most fundamental concepts in chemistry, has attracted considerable attention from both theoreticians and experimentalists. Much effort on aromaticity in organometallics has been devoted to metallabenzene and derivatives. In comparison, aromaticity in other organometallics is less developed. This Account describes how our group has performed quantum chemical calculations to examine aromaticity in recently synthesized novel organometallic complexes.
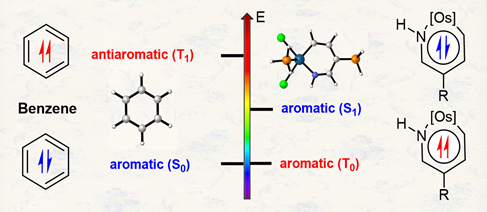
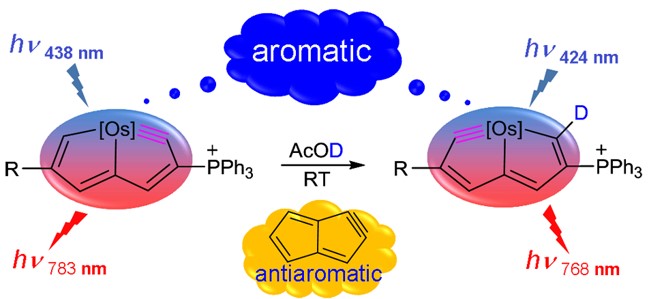
According to Hückel’s and Baird’s rules, cyclic conjugated species are aromatic either in the ground state or in the excited state only. Thus, species with aromaticity in both states (denoted as adaptive aromaticity) are particularly rare. Here we carry out density functional theory calculations on a series of osmapyridine and osmapyridinium complexes (96 species) and find that two of them display adaptive aromaticity, which was verified by various aromaticity indices including HOMA, ELFπ, MCI, ACIDπ plots and the heat of hydrogenation.
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. Although it has been extensively investigated in various reactions, the regioselectivity of hyperconjugative aromaticity on either main group systems or transition metal ones remains elusive due to the challenge of synthesizing the target products. Here we report a joint theoretical and experimental study on this issue.
Copyright © 2026,
Theme Originally Created by Devsaran
