Nature Chemistry



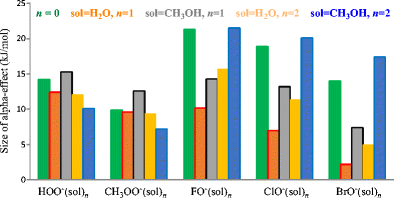
The modified G4(MP2) method was applied to explore microsolvation effects on the reactivity of four solvated normal oxy-nucleophiles YO−(CH3OH)n=1,2 (Y = CH3, C2H5, FC2H4, ClC2H4), and five α-oxy-nucleophiles YO−(CH3OH)n=1,2 (Y = HO, CH3O, F, Cl, Br), in gas-phase SN2 reactions towards the substrate CH3Cl.
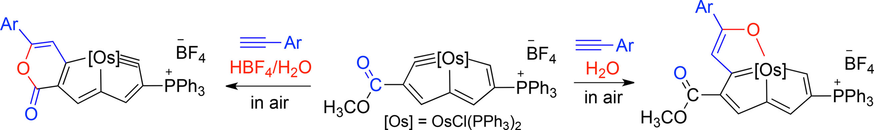
Metalla-aromatics are attractive species because they exhibit the properties of both organometallics and aromatics. Reported metal-bridged polycyclic aromatic complexes, as well as Möbius aromatic species, are still rare. Herein, we present the construction of two new metal-bridged polycyclic aromatic frameworks, α-metallapentalenofurans and lactone-fused metallapentalynes, by the reactions of osmapentalyne with terminal aryl alkynes in the presence of H2O or HBF4/H2O, respectively.

The BN-doped organic analogues are interesting as aliphatic amineboranes for hydrogen storage, precursors for aromatic borazines and adsorbent cage azaboranes. However, BN-doped aliphatic polyenes remained undeveloped. Herein, we perform theoretical calculations on two mono BN-doped aliphatic lower polyenes, 1,3-butadiene and 1,3,5-hexatriene. A general rule is proposed, i.e., isomers with terminal nitrogen and directly BN-connected, N−B(R), in particular, are of significant thermodynamic stability as compared with their inverse analogues (where boron is at the terminal position).
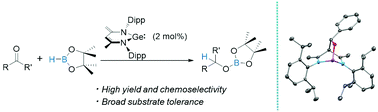
N-heterocyclic ylide-like germylene effectively promotes the hydroboration of aldehydes and ketones under mild conditions with broad substrate tolerance, operational simplicity of procedure and excellent yields. A key intermediate in this catalytic system featuring a bicyclo[2,2,2]octane-like core has been successfully isolated and characterized, suggesting a new type of mechanism that involves the activation mode that mimics that of transition metal catalysts.
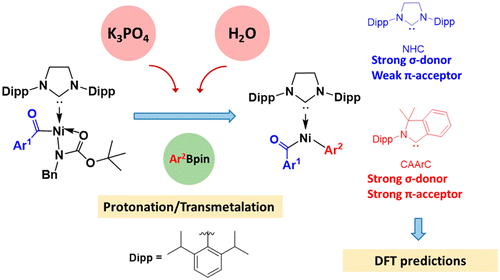
In textbooks, the low reactivity of amides is attributed to the strong resonance stability. However, Garg and co-workers recently reported the Ni-catalyzed activation of robust amide C–N bonds, leading to conversions of amides into esters, ketones, and other amides with high selectivity. Among them, the Ni-catalyzed Suzuki-Miyaura coupling (SMC) of N-benzyl-N-tert-butoxycarbonyl (N-Bn-N-Boc) amides with pinacolatoboronate (PhBpin) was performed in the presence of K3PO4 and water. Water significantly enhanced the reaction.

The coordinating atoms in polydentate chelates are primarily heteroatoms. We present the first examples of pentadentate chelates with all binding atoms of the chelating agent being carbon atoms, denoted as CCCCC chelates. Having up to five metal-carbon bonds in the equatorial plane has not been previously observed in transition metal chemistry. Density functional theory calculations showed that the planar metallacycle has extended Craig-Möbius aromaticity arising from 12-center–12-electron dπ-pπ π-conjugation.

Metallabenzenes have attracted considerable interest of both theoretical and experimental chemists. However, metallaphosphabenzene has never been synthesized. Thus, understanding the origin of the challenge of synthesizing metallaphosphabenzene is particularly urgent for experimentalists. Now density functional theory (DFT) calculations have been carried out to examine this issue.
Polymetalated aromatic compounds are particularly challenging synthetic goals because of the limited thermodynamic stability of polyanionic species arising from strong electrostatic repulsion between adjacent carbanionic sites. Here we describe a facile synthesis of two polyaurated complexes including a tetra-aurated indole and an octa-aurated benzodipyrrole. The imido trinuclear gold(I) moiety exhibits nucleophilicity and undergoes an intramolecular attack on a gold(I)-activated ethynyl to generate polyanionic heteroaryl species.
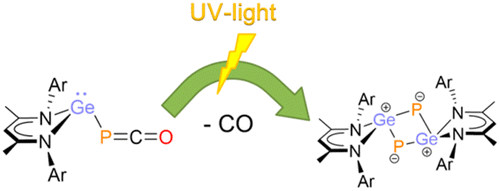
The heavier cyclobutadiene analogue 2,4-digerma-1,3-diphosphacyclobutadiene ([L12Ge2P2], 4; L1 = CH{(CMe)(2,6-iPr2C6H3N)}2), featuring a planar Ge2P2 four-membered ring, has been synthesized via the elimination of carbon monoxide from the corresponding phosphaketenyl germylene [L1GePCO] (2) under UV irradiation.
DOI: 10.1039/C6QO90012G

http://pubs.rsc.org/en/content/articlelanding/2016/qo/c6qo90012g#!divAbstract
Copyright © 2026,
Theme Originally Created by Devsaran
