Nature Chemistry


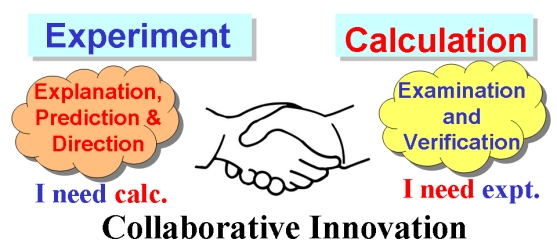

Density functional theory (DFT) calculations were carried out to investigate the 1,2-migration in metallasilabenzenes. The results suggested that the chloride migration of metallabenzenes is unfavorable due to the loss of aromaticity in the nonaromatic analogues. In sharp contrast, such a migration in metallasilabenzenes is favorable due to the reluctance of silicon to participate in π bonding. The migration of hydride and methyl group from the metal center to the silicon atom in metallasilabenzenes is computed to be also feasible.
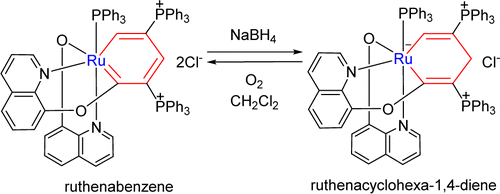
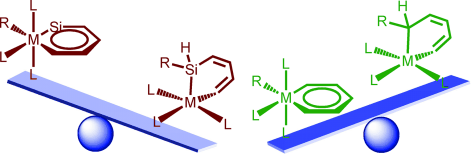
Treatment of ruthenabenzene [(C9H6NO)Ru{CC(PPh3)CHC(PPh3)CH}(C9H6NO)(PPh3)]Cl2 (1) with NaBH4 produces the first ruthenacyclohexa-1,4-diene [(C9H6NO)Ru{CC(PPh3)CH2C(PPh3)CH}(C9H6NO)(PPh3)]Cl (2), which was fully characterized. Under an oxygen atmosphere, complex 2 can easily convert to complex 1. DFT calculations were carried out to rationalize the high regioselectivity in the reaction of the ruthenabenene 1 with NaBH4 and the interconversion between 1 and 2.
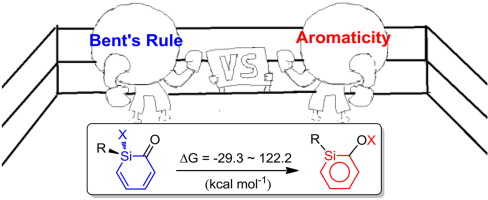
Density functional theory (DFT) calculations were performed to examine the substituent effects on the interconversion of silabenzenes and their monocyclic non-aromatic isomers. A previous study suggested that aromaticity is the driving force for this process. Interestingly, our systematic calculations reveal that the contribution from aromaticity can be evaluated quantitatively (ca. 30 kcal mol-1). Thus it is the interplay of aromaticity and Bent's rule that determine their relative stabilities.
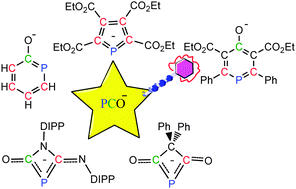
Density functional theory (DFT) calculations were carried out to investigate the [2+2], [3+2] and [4+2] cycloadditions of the phosphaethynolate anion (PCO−). The results reveal that the electronic properties of different unsaturated compounds play a crucial role in reactivity and regioselectivity.
http://pubs.rsc.org/en/Content/ArticleLanding/2014/CC/C4CC04610B#!divAbstract
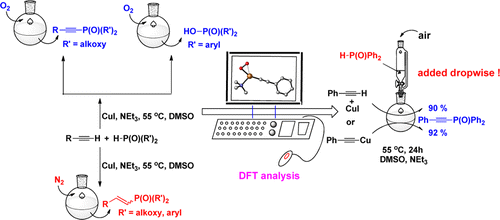
The reaction mechanism of copper-catalyzed phosphorylation of terminal alkynes under different conditions has been investigated experimentally and theoretically. The important role of dioxygen has been elucidated, including the formation of η1-superoxocopper(II), η2-superoxocopper(III), μ-η2:η2-peroxodicopper(II), and bis(μ-oxo)dicopper(III) complexes.
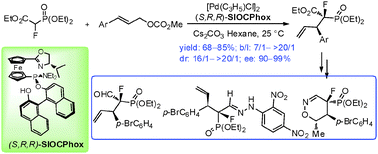
Highly efficient Pd-catalyzed asymmetric allylic alkylation reaction of ethyl-2-fluoro-2-(diethoxyphosphoryl)acetate with monosubstituted allylic substrates has been developed, affording corresponding α-fluorophosphonates with two chiral centers in high regio-, diastereo- and enantio-selectivities. The usefulness of the products in organic synthesis has been demonstrated.
http://pubs.rsc.org/en/content/articlelanding/2014/cc/c4cc02158d#!divAbstract
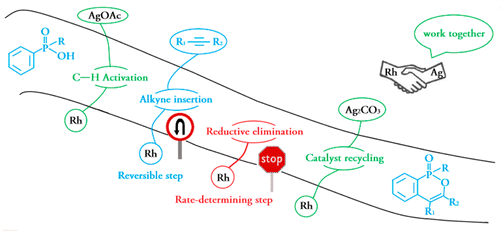
Density functional theory calculations (DFT) have been performed on Rh(III)-catalyzed phosphoryl-directed oxidative C–H activation/cyclization to investigate the detailed mechanism, including four basic steps: C–H activation, alkyne insertion, reductive elimination, and catalyst recycling, each of which consists of different steps. Interestingly, the Rh(III)–AgOAc catalyst system was found to be more favorable in the C–H activation step in comparison with the Rh(III)–Ag2CO3 system, whereas the Rh(I)–Ag2CO3 catalyst system was more efficient for catalyst recycling.
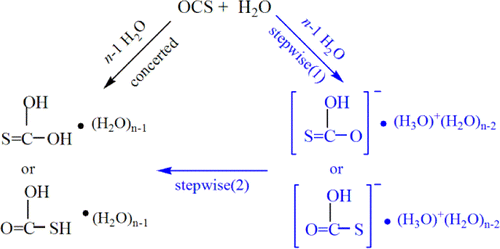
The water-mediated neutral hydrolysis mechanism of carbonyl sulfide (OCS) has been re-examined using the hybrid supramolecule/continuum models with n = 2–8 explicit water cluster at the level of MP2(fc)(CPCM)/6-311++G(d,p)//MP2(fc)(CPCM) /6-31+G(d). Present calculations indicate that the potential energy surface in water solution is different from the one in the gas-phase, and only stepwise mechanism is observed in aqueous solution, i.e., monothiocarbonic acid (H2CO2S) is formed via monothiocarbonate (OCSOH–, MTC) and its counterion, protonated water cluster, (H2O)nH3O+.
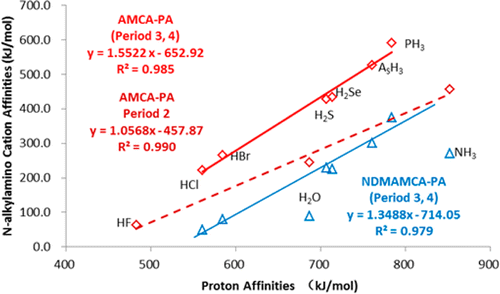
We have made an extensive theoretical exploration of gas-phase N-alkylamino cation affinities (NAAMCA), including amino cation affinities (AMCA) and N-dimethylamino cation affinities (NDMAMCA), of neutral main-group element hydrides of groups 15–17 and periods 2–4 in the periodic table by using the G2(+)M method. Some similarities and differences are found between NAAMCA and the corresponding alkyl cation affinities (ACA) of HnX.
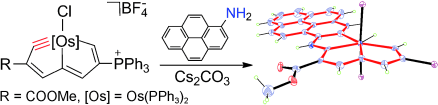
Aromaticity is one of the most important concepts in organic chemistry. A variety of metalla-aromatic compounds have been recently prepared and in most of those examples, the metal participates only in a monocyclic ring. In contrast, metal-bridged bicyclic aromatic molecules, in which a metal is shared between two aromatic rings, have been less developed. Herein, we report the first metal-bridged tricyclic aromatic system, in which the metal center is shared by three aromatic five-membered rings. These metalla-aromatics are formed by reaction between osmapentalyne and arene nucleophiles.
Copyright © 2026,
Theme Originally Created by Devsaran
