Double Role of the Hydroxy Group of phosphoryl in Palladium(II)-catalyzed ortho-Olefination: A Combined Experimental and Theoretical Investigation
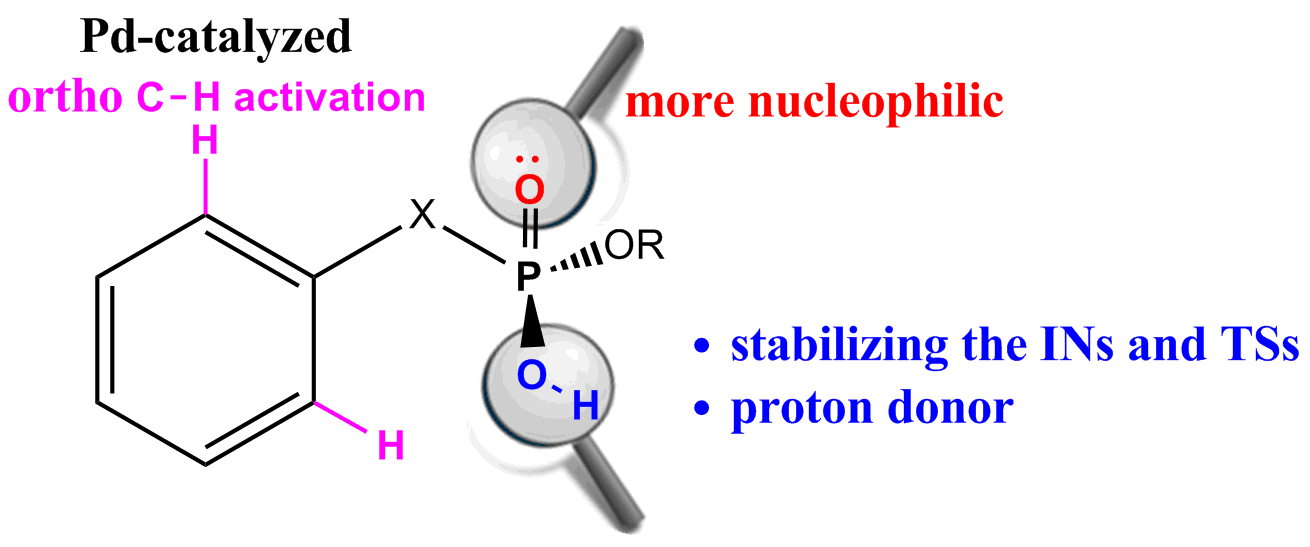
Density functional theory (DFT) calculations have been carried out on Pd-catalyzed phosphoryl-directed ortho-olefination to probe the origin of the significant reactivity difference between methyl hydrogen benzylphosphonates and dialkyl benzylphosphonates. The overall catalytic cycle is found to include four basic steps: C−H bond activation, transmetalation, reductive elimination and recycling of catalyst, each of which is constituted from different steps. Our calculations reveal that the hydroxy group of phosphoryl plays a crucial role almost in all steps, which can not only stabilize the intermediates and transition states by intramolecular hydrogen bonds, but also act as a proton donor so that the η1-CH3COO− ligand could be protonated to form a neutral acetic acid for easy removal. These findings explain why only the methyl hydrogen benzylphosphonates and methyl hydrogen phenylphosphates were found to be suitable reaction partners. Our mechanistic findings are further supported by theoretical prediction of Pd-catalyzed ortho-olefination using methyl hydrogen phenylphosphonate which is verified by experimental observations that the desired product was formed in a moderate yield.
