Nature Chemistry



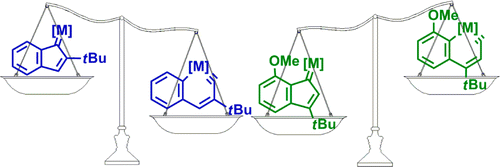
Metallaaromatics have attracted considerable interest of both theoretical and experimental chemists. However, there have been only two metallanaphthalynes isolated so far. Thus, developing new synthetic approaches is urgent. Here we present thorough density functional theory (DFT) calculations on the thermodynamics and kinetics of the isomerization between metallanaphthalynes and metal indenylidene complexes. The effects of metal centers, ligands, and substituents on the metallabicycles were examined systematically.
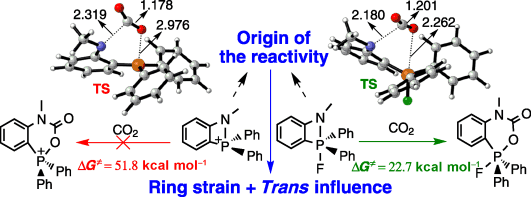
CO2 capture has attracted increasing attention owing to its contribution to global warming and climate change as a greenhouse gas. As an alternative strategy to transition-metal-based chemistry and catalysis, frustrated Lewis pairs have been developed to sequester CO2 efficiently under mild conditions. However, the mechanism of CO2 sequestration with amidophosphoranes remains unclear. Herein, we present a thorough density functional theory study on a series of amidophosphoranes.
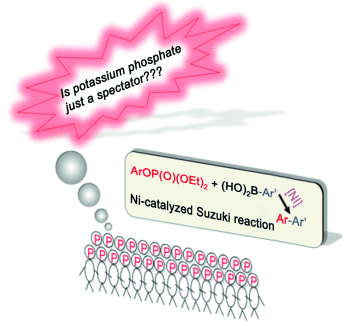
Spectator or actor? Density functional theory calculations were performed to examine the role of the base in the nickel-catalyzed cross-coupling of aryl phosphates with arylboronic acids. Potassium phosphate was found to not act as a spectator base but was involved in the transmetalation step, as shown by a lower barrier than that of a base-free process, owing to the activation of the carbonboron bond by the base. Further experimental observations support the theoretical findings.
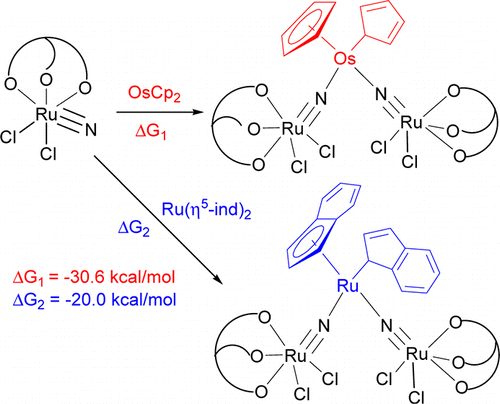
η5–η1 ring slippage of [OsCp2] (Cp = η5-C5H5) and [Ru(η5-ind)2] (ind = indenyl) resulting from reaction with the ruthenium(VI) nitride [Ru(LOEt)(N)Cl2] (1; LOEt– = [CoCp{P(O)(OEt)2}3]−) is reported. The treatment of [OsCp2] or [Ru(η5-ind)2] with 1 resulted in η5-η1 ring slippage of the cycloolefin ligands and formation of the trinuclear nitrido complexes [Cp(η1-C5H5)Os(NRuLOEtCl2)2] (2) or [(η5-ind)(η1-ind)Ru(NRuLOEtCl2)2] (3).
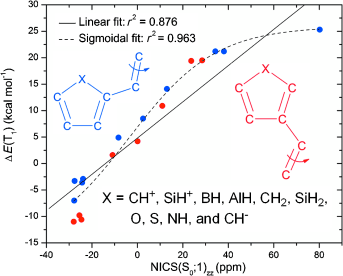
A density functional theory study on olefins with five-membered monocyclic 4n and 4n+2 π-electron substituents (C4H3X; X=CH+, SiH+, BH, AlH, CH2, SiH2, O, S, NH, and CH−) was performed to assess the connection between the degree of substituent (anti)aromaticity and the profile of the lowest triplet-state (T1) potential-energy surface (PES) for twisting about olefinic CC bonds. It exploited both Hückel’s rule on aromaticity in the closed-shell singlet ground state (S0) and Baird’s rule on aromaticity in the lowest ππ* excited triplet state.
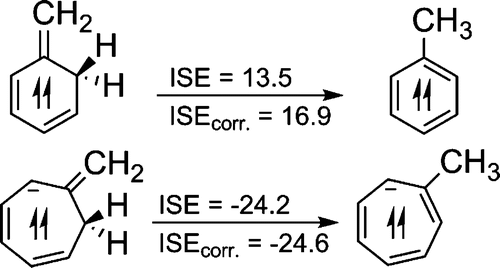
The many manifestations of aromaticity have long fascinated both experimentalists and theoreticians. Due to their degenerate half-filled MOs, triplet [n]annulenes with 4n π-electrons are also aromatic, but the degree of their stabilization has been difficult to quantify. The isomerization stabilization energy (ISE) method has been applied to evaluate the triplet aromaticity. The reliability of this approach is indicated by the strong correlation of the ISE results with NICS(1)zz, a magnetic indicator of triplet state aromaticity.

A one-pot synthesis of 3,4,5- and 1,3,5-pyrazoles from tertiary propargylic alcohols and para-tolylsulfonohydrazide has been accomplished. The pyrazoles are formed through a four-step cascade sequence, including FeCl3-catalyzed propargylic substitution, aza-Meyer–Schuster rearrangement, base-mediated 6π electrocyclization, and thermal [1,5] sigmatropic shift. In this reaction, the 3,4,5- and 1,3,5-pyrazoles are produced selectively according to different substituents in the starting alcohols.
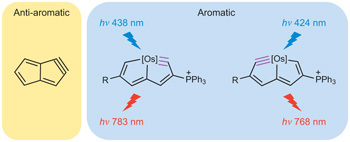
Anti-aromatic compounds, as well as small cyclic alkynes or carbynes, are particularly challenging synthetic goals. The combination of their destabilizing features hinders attempts to prepare molecules such as pentalyne, an 8π-electron anti-aromatic bicycle with extremely high ring strain. Here we describe the facile synthesis of osmapentalyne derivatives that are thermally viable, despite containing the smallest angles observed so far at a carbyne carbon.

We report herein the first example of the conversion of metallabenzyne II and isometallabenzene III. The osmium hydride vinylidene complex 1 reacts with HCCCH(OEt)2 to give osmabenzyne 3 via isoosmabenzene 2. Compound 3 exhibits high thermal stability in air. Nonetheless, nucleophilic attack at 3 provides isoosmabenzenes 4 a and 4 b, or opens the ring to produce 5 a and 5 b.
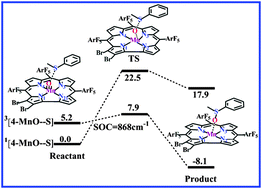
The electronic and structural features of (oxo)manganese(V) corroles and their catalyzed oxygen atom transfers to thioanisole in different spin states have been investigated by the B3LYP functional calculations. Calculations show that these corrole-based oxidants and their complexes with thioanisole generally have the singlet ground state, and their triplet forms are also accessible in consideration of the spin–orbit coupling interaction.
Copyright © 2026,
Theme Originally Created by Devsaran
