Nature Chemistry



Metallaaromatics have attracted considerable interest from both experimentalists and theoreticians over the past three decades. However, most studies in this field have focused on metallabenzene, in which a CH group is replaced by a transition metal fragment. In comparison with monocyclic metallabenzenes, bicyclic metallanaphthalenes are rather limited. Thus, it is urgent to explore more synthetic approaches to this less developed system. One of the difficulties in the synthesis of metallanaphthalenes could be due to its low thermodynamic stability relative to the metal indenyl complexes.
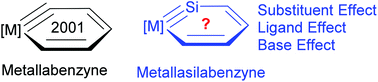
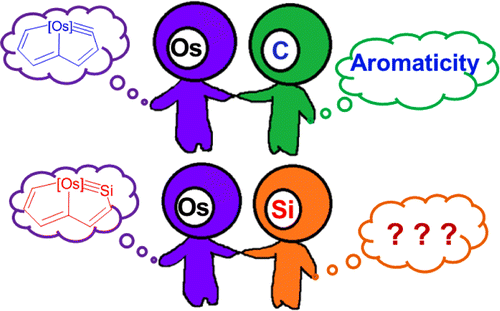
Metallabenzyne has attracted considerable interest from theoreticians and experimentalists since its first isolation in 2001. However, metallasilabenzyne, formed by the replacement of the carbyne carbon with a silicon atom in metallabenzyne, has never been reported either theoretically or experimentally. Here we carry out density functional theory (DFT) calculations on this system for the first time. Our results reveal a polarized and weak Os–Si triple bond in osmasilabenzyne due to the reluctance of the silicon to participate in π bonding.
Antiaromatic compounds and small cyclic alkynes or carbynes are both challenging for synthetic chemists because of the destabilization caused by their antiaromaticity and highly distorted triple bonds, respectively. These dual destabilizations could be the reason why pentalyne (I), a highly antiaromatic and extremely strained cyclic alkyne, has never been synthesized.
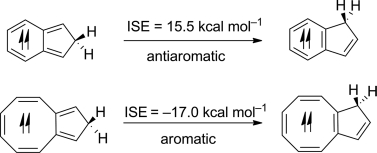
Aromaticity, one of the most important concepts in chemistry, has attracted considerable interest from both experimentalists and theoreticians. According to Baird's rule, triplet annulenes with 4n π electrons are aromatic. However, the approach to evaluate the magnitude of the triplet aromaticity is less developed. Herein we apply the indene–isoindene isomerization stabilization energy (ISE) method to evaluate the aromaticity in the triplet state.
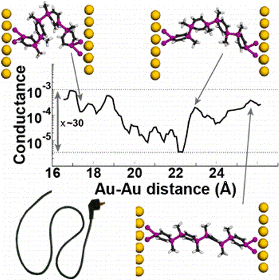
Oligomers of 1,4-disila/germa/stannacyclohexa-2,5-dienes as well as all-carbon 1,4-cyclohexadienes connected via E—E single bonds (E = C, Si, Ge, or Sn) were studied through quantum chemical calculations in an effort to identify conformationally flexible molecular wires that act as molecular “electrical cords” having conformer-independent conjugative and conductive properties. Our oligomers display neutral hyperconjugative interactions (σ/π-conjugation) between adjacent σ(E—E) and π(C═C) bond orbitals, and these interactions do not change with conformation.

Aromaticity, a highly stabilizing feature of molecules with delocalized electrons in closed circuits, is generally restricted to ‘Hückel’ systems with 4n+2 mobile electrons. Although the Möbius concept extends the principle of aromaticity to 4n mobile electron species, the rare known examples have complex, twisted topologies whose extension is unlikely. Here we report the realization of osmapentalenes, the first planar Möbius aromatic complexes with 16 and 18 valence electron transition metals.
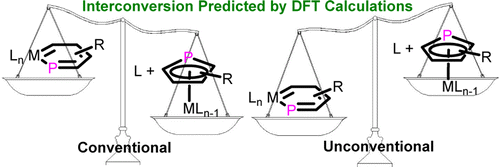
Metallaaromatics have attracted continuing interest of both theoretical and experimental chemists since the first metallabenzene was predicted by Hoffmann and isolated by Roper. In sharp contrast to metallabenzenes, metallaphosphabenzene (MPB) is much less developed and has not been synthesized so far. Thus, developing synthetic approaches is urgent. Here we present thorough density functional theory (DFT) calculations on the thermodynamics and kinetics of the rearrangement between MPBs and the corresponding η5-phosphacyclopentadiene (η5-PCp) complexes.
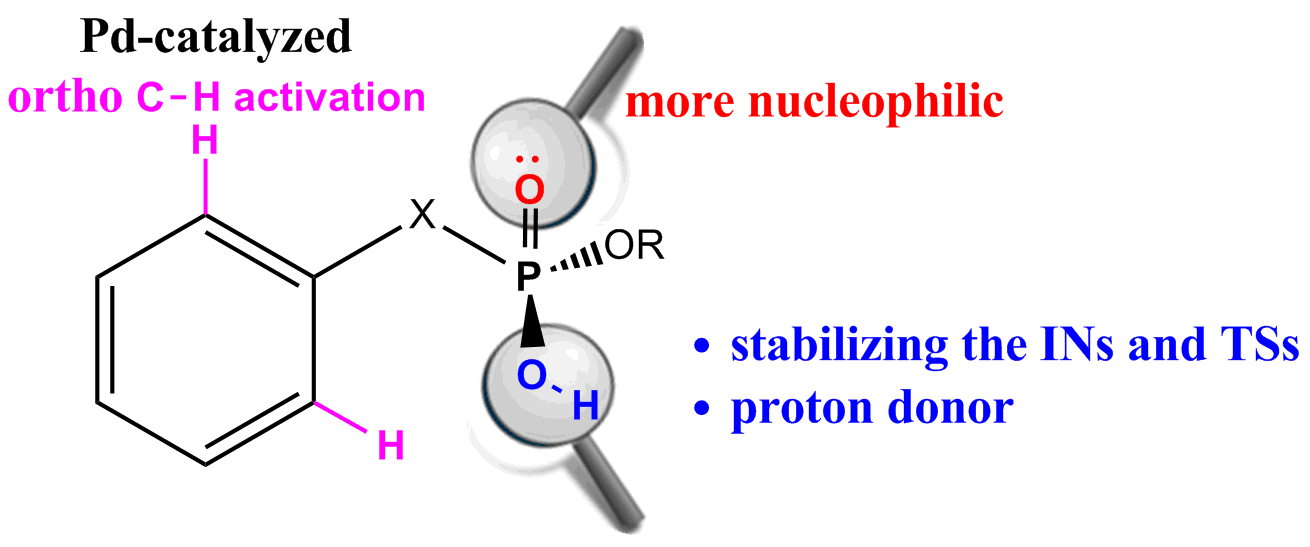
Density functional theory (DFT) calculations have been carried out on Pd-catalyzed phosphoryl-directed ortho-olefination to probe the origin of the significant reactivity difference between methyl hydrogen benzylphosphonates and dialkyl benzylphosphonates. The overall catalytic cycle is found to include four basic steps: C−H bond activation, transmetalation, reductive elimination and recycling of catalyst, each of which is constituted from different steps.
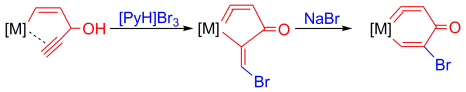
Highly stable five-membered metallacycloallenes were synthesized under mild conditions. Calculations revealed that the incorporation of transition-metal moieties relieves considerable strain and indicates a trend toward ring enlargement in the five-membered metallacycloallenes. Conversion into six-membered metallacycloallenes was confirmed experimentally.
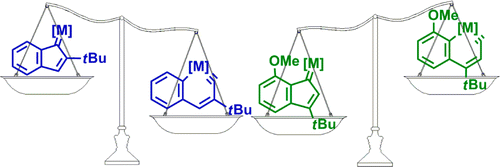
Metallaaromatics have attracted considerable interest of both theoretical and experimental chemists. However, there have been only two metallanaphthalynes isolated so far. Thus, developing new synthetic approaches is urgent. Here we present thorough density functional theory (DFT) calculations on the thermodynamics and kinetics of the isomerization between metallanaphthalynes and metal indenylidene complexes. The effects of metal centers, ligands, and substituents on the metallabicycles were examined systematically.
Copyright © 2026,
Theme Originally Created by Devsaran
