Significant effect of spin flip on the oxygen atom transfer reaction from (oxo)manganese(v) corroles to thioanisole: insights from density functional calculations
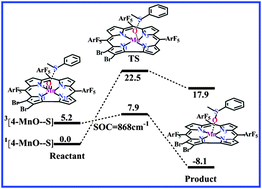
The electronic and structural features of (oxo)manganese(V) corroles and their catalyzed oxygen atom transfers to thioanisole in different spin states have been investigated by the B3LYP functional calculations. Calculations show that these corrole-based oxidants and their complexes with thioanisole generally have the singlet ground state, and their triplet forms are also accessible in consideration of the spin–orbit coupling interaction. Due to strong d–π conjugation interactions between Mn and the corrole ring arising from the π electron donation of the corrole moiety, the five-coordinated Mn approximately has the stable 18-electron configuration. The predicted free energy barriers for the singlet oxygen atom transfer reactions are generally larger than 22 kcal mol−1, while the spin flip in reaction may remarkably increase the reactivity. In particular, the bromination on β-pyrrole carbon atoms of the meso-substituted (oxo)manganese(V) corrole strikingly enhances the spin–orbit coupling interaction and results in the dramatic increase of reactivity. The multiple spin changes are predicted to be involved in the low-energy reaction pathway. The present results show good agreement with the experimental observation and provide a detailed picture for the oxygen atom transfer reaction induced by the (oxo)manganese(V) corroles.
