Evaluation of Triplet Aromaticity by the Indene–Isoindene Isomerization Stabilization Energy Method
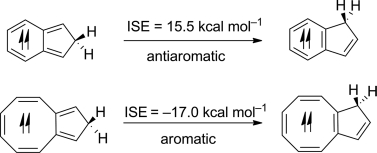
Aromaticity, one of the most important concepts in chemistry, has attracted considerable interest from both experimentalists and theoreticians. According to Baird's rule, triplet annulenes with 4n π electrons are aromatic. However, the approach to evaluate the magnitude of the triplet aromaticity is less developed. Herein we apply the indene–isoindene isomerization stabilization energy (ISE) method to evaluate the aromaticity in the triplet state. The reliability of this approach can be demonstrated by the strong correlation of these indene–isoindene ISE values with nucleus-independent chemical shifts [NICS(1)zz] as well as methyl–methylene ISE values. Large [4n]annulenes have the tendency to be planar to achieve aromaticity in the T1 state. Steric effects play an important role in the stabilities of large [4n]annulene isomers.
http://onlinelibrary.wiley.com/doi/10.1002/ejoc.201301810/abstract
