FP(μ-N)2S: A Sulfur-Pnictogen Four-Membered Ring with 6π Electrons
Submitted by Jun Zhu on Fri, 06/23/2023 - 09:25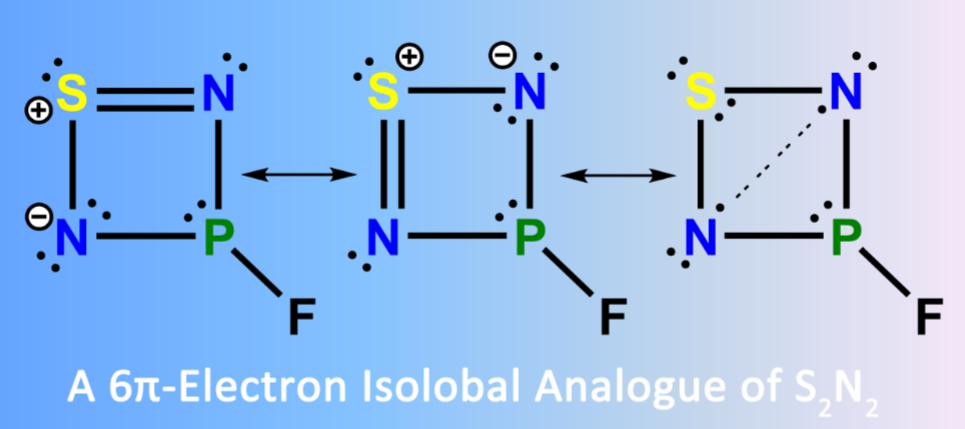
The new 6π-electron four-membered ring compound 3-fluoro-1λ2,2,4,3λ3-thiadiazaphosphetidine, FP(μ-N)2S, has been generated in the gas phase through high-vacuum flash pyrolysis (HVFP) of thiophosphoryl diazide, FP(S)(N3)2, at 1000 K. Subsequent isolation of FP(μ-N)2S in cryogenic matrices (Ar, Ne, and N2) allows its characterization with matrix-isolation IR and UV-vis spectroscopy by combination with 15N-isotope labeling and computations at the CCSD(T)-F12a/VTZ-F12 level of theory.