Nature Chemistry




Hyperconjugation and aromaticity are two of the most important concepts in chemistry. Mulliken and co‐workers combined both terms to explain the stability of cyclopentadiene. Here, we carried out DFT calculations on a series of mono‐ and disubstituted cyclopentadiene derivatives to investigate their hyperconjugative aromaticity. Our results revealed that one electropositive substituent can induce aromaticity, whereas one electronegative substituent prompts nonaromaticity rather than antiaromaticity.

Aromaticity is one of the most fundamental and fascinating chemical topics, attracting both experimental and theoretical chemists owing to its many manifestations. Both σ‐ and π‐aromaticity can be classified depending on the character of the cyclic electron delocalization. In general, σ‐aromaticity stabilizes fully saturated rings with σ‐electron delocalization whereas the traditional π‐aromaticity describes the π‐conjugation in fully unsaturated rings.

Rational design of a molecular catalyst for a hydrogen evolution reaction (HER) with both high rates and low overpotential remains a challenge. Here we report a series of Ni‐based catalysts incorporating non‐innocent ligands and aimed at reduction of the overpotential by reserving electrons in the ligand, which could decouple the correlation between metal center reduction and metal hydride formation.
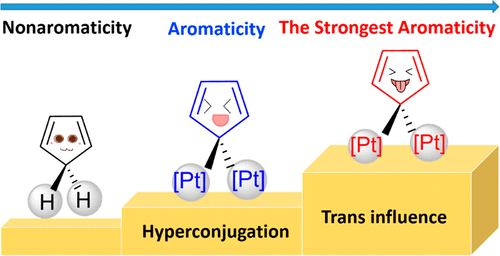
Hyperconjugation, an interaction of electrons in a σ orbital or lone pair with an adjacent π or even σ antibonding orbital, can have a strong effect on aromaticity. However, most work on hyperconjugative aromaticity has been limited to main-group substituents. Here, we report a thorough density functional theory study to evaluate the aromaticity in various cyclopentadienes that contain both main-group and transition-metal substituents.

Besides its mathematical importance, the Möbius topology (twisted, single-sided strip) is intriguing at the molecular level, as it features structural elegance and distinct properties; however, it carries synthetic challenges. Although some Möbius-type molecules have been isolated by synthetic chemists accompanied by extensive computational studies, the design, preparation, and characterization of stable Möbius-conjugated molecules remain a nontrivial task to date, let alone that of molecular Möbius strips assembling into more complex topologies.
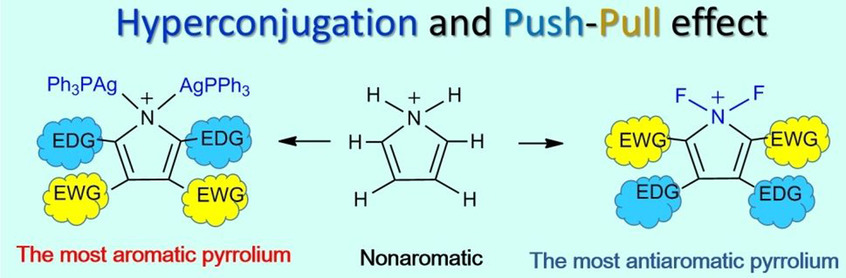
Hyperconjugation, a weak interaction in organic chemistry, can have a strong effect on aromaticity, leading to the concept of hyperconjugative aromaticity, which was first proposed by Mulliken in 1939. However, most studies are limited to main group chemistry. Here we report the most aromatic and antiaromatic pyrrolium ring by maximizing the hyperconjugation caused by transition metal fragments and the push–pull effect.
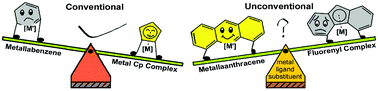
Metallaaromatics have attracted considerable interest from both experimentalists and theoreticians since the first prediction of metallabenzenes, in which a CH group is replaced by a transition metal fragment. In comparison with monocyclic metallabenzenes and bicyclic metallanaphthalenes, tricyclic metallaanthracenes are quite less developed. Thus, it is urgent to explore synthetic methods for this rare system. Here we report a thorough investigation on the formation of metallaanthracenes from transition metal fluorenyl complexes via density functional theory calculations.
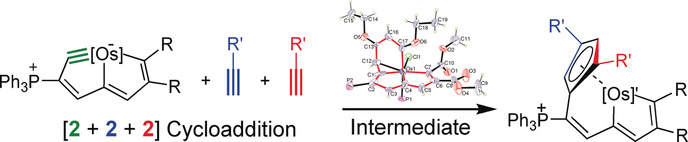
Carbon ligands have long played an important role in organometallic chemistry. However, previous examples of all‐carbon chelating ligands are limited. Herein, we present a novel complex with an eleven‐atom carbon chain as a polydentate chelating ligand. This species was formed by the [2+2+2] cycloaddition reaction of two equivalents of an alkyne with an osmapentalyne that contains the smallest carbyne bond angle (127.9°) ever observed. Density functional calculations revealed that electron‐donating groups play a key role in the stabilization of this polydentate carbon‐chain chelate.
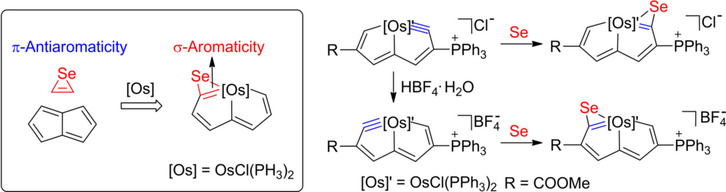
Isolation of the simplest 4π three‐membered heterocycles (1H‐azirine, oxirene, thiirene, and selenirene) remains a big challenge due to their π‐antiaromaticity and significant ring strain. Here we demonstrate that the incorporation of a transition‐metal fragment could stabilize the antiaromatic selenirene and pentalene frameworks simultaneously by density functional theory (DFT) calculations. Experimental verification leads to the Se‐containing metallapolycycles, osmapentaloselenirenes, with remarkable thermal stability.
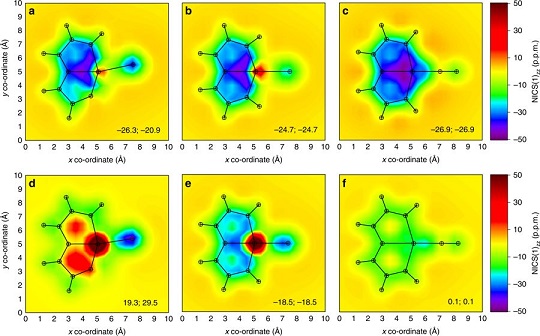
Aromaticity is a fundamental chemical concept of ever-increasing diversity. According to Hückel’s and Baird’s rules, cyclic conjugated species with 4n+2 π-electrons are aromatic in the singlet electronic ground state (S0) and antiaromatic in the lowest triplet state (T1), and vice-versa. Thus, species with aromaticity in both states have not yet been reported.
Copyright © 2026,
Theme Originally Created by Devsaran
