Microsolvation effects on the reactivity of oxy-nucleophiles: the case of gas-phase SN2 reactions of YO-(CH3OH) n=1,2 towards CH3Cl
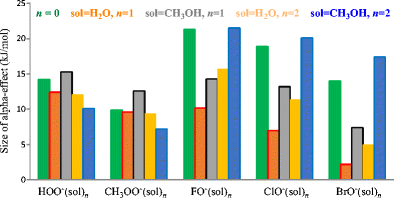
The modified G4(MP2) method was applied to explore microsolvation effects on the reactivity of four solvated normal oxy-nucleophiles YO−(CH3OH)n=1,2 (Y = CH3, C2H5, FC2H4, ClC2H4), and five α-oxy-nucleophiles YO−(CH3OH)n=1,2 (Y = HO, CH3O, F, Cl, Br), in gas-phase SN2 reactions towards the substrate CH3Cl. Based on a Brønsted-type plot, our calculations reveal that the overall activation barriers of five microsolvated α-oxy-nucleophiles are obviously smaller than the prediction from the correlation line constructed by four normal microsolvated ones to different degrees, and clearly demonstrate the existence of an α-effect in the presence of one or two methanol molecule(s). Moreover, it was found that the α-effect of the mono-methanol microsolvated α-nucleophile is stronger than that of the monohydrated α-nucleophile. However, the α-effect of YO−(CH3OH)2 becomes weaker for Y = HO and CH3O, whereas it becomes stronger for Y = F, Cl, Br than that of YO−(H2O)2, which can be explained by analyses of the activation strain model in the two cases. It was also found that the rationale about the low ionization energy of α-nucleophile inducing the α-effect was not widely significant.
