To Be Bridgehead or Not to Be? This is a Question of Metallabicycles on the Interplay between Aromaticity and Ring Strain
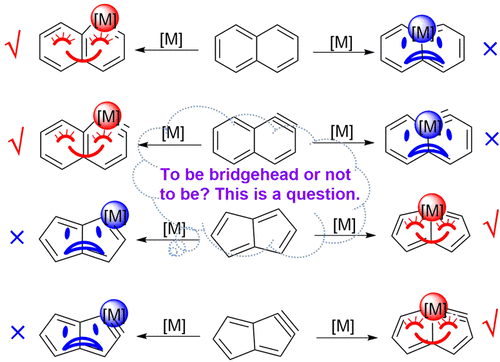
Transition-metal-containing metallaaromatics have attracted considerable interest from both experimental and computational chemists because they can display properties of both organometallic compounds and aromatic organic compounds. In general, the transition metal in a metallabicycle prefers a nonbridged position to the bridgehead one because of the larger ring strain caused by the rigidity in the bridgehead position, as exemplified by metallanaphthalene and metallanaphthalyne. On the contrary, the osmium atoms in recently synthesized osmapentalyne and osmapentalene are located at the bridgehead position. To probe the origin of the difference between these metallabicycles, we carried out density functional theory calculations. The metal-bridgehead osmanaphthalene and osmanaphthalyne are computed to be less stable by 17.9 and 26.3 kcal mol–1 than the non-metal-bridged ones, respectively. In addition, the metal-bridgehead osmapentalene and osmapentalyne are more stable by 11.8 and 22.8 kcal mol–1 than the non-metal-bridged isomers, respectively. Further study revealed that the ring strains in these paired isomers are comparable to each other. Thus, it is aromaticity rather than ring strain that determines the relative thermodynamic stabilities of these complexes.
