Nature Chemistry



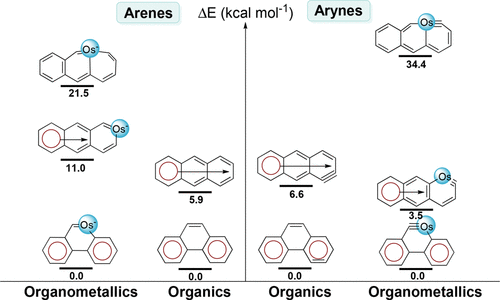
Metallaaromatics have attracted considerable attention in recent years because they can display properties of both organic and organometallic species. However, it remains unclear whether Clar’s rule could be applied to organometallic chemistry despite its proposal in 1950s. Here, we investigate the relative stabilities of 49 organic and organometallic species by density functional theory (DFT) calculations.
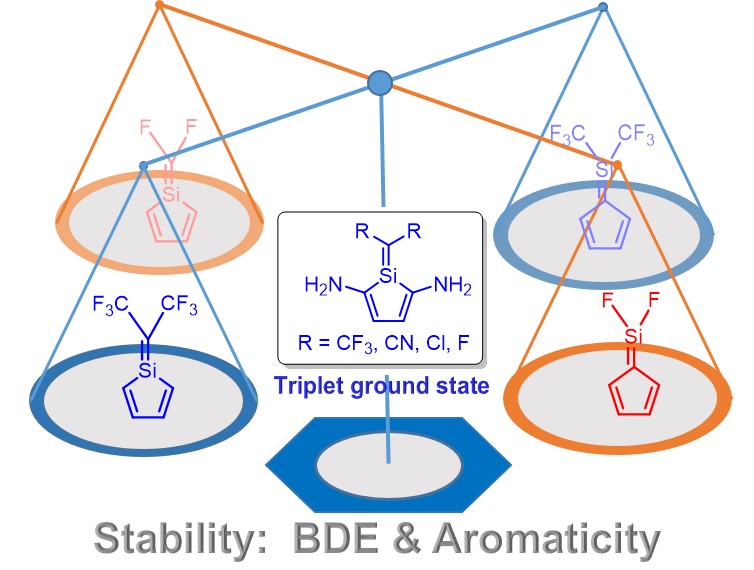
Pentafulvenes are dipolar hydrocarbons since they shift their π-electrons to achieve Hückel aromaticity and thus the electron donating groups at the exocyclic position can enhance their aromaticity. Silapentafulvenes are analogues of pentafulvene formed by the replacement of the carbon atoms at the exocyclic CC double bond with a silicon atom in pentafulvene. It remains unclear how the aromaticity of 5-silapentafulvenes and 6-silapentafulvenes can be changed due to the polarization of the CSi double bond.
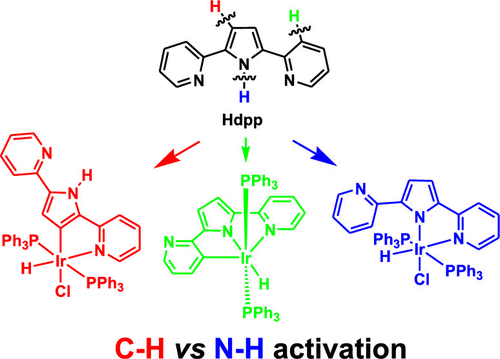
Treatment of [Ir(PPh3)3Cl] with 2-[5-(pyridin-2-yl)-1H-pyrrol-2-yl]pyridine (Hdpp) in refluxing toluene affords an unexpected pyrrole-metalated iridium(III) hydride complex, [Ir(K2C,N-dpp)(H)(Cl)(PPh3)2] (1), via Cpyrrole–H activation, while the presence of the base KOtBu as the deprotonation reagent produces a pyridine-metalated iridium(III) hydride complex, [Ir(K3C,N,N-dpp)(H)(PPh3)2] (2), via Cpyridine–H activation.
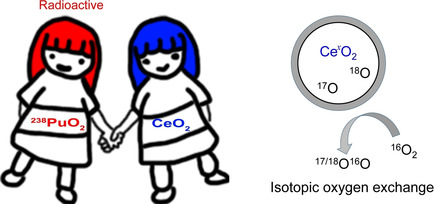
Isotopic oxygen exchange (IOE) is a crucial reaction required in the purification of 238PuO2 which has been used as an important fuel in space exploration. Experimental studies on the IOE between 238PuO2 and O2 are costly and hazardous due to the radioactivity. With extremely similar crystal structures, CeO2 could be a fair surrogate for 238PuO2 in the investigation of physicochemical properties. Here, we perform density functional theory calculations to simulate the IOE between CeO2 and O2, wherein a heteroexchange mechanism is proposed.

Carbon dioxide (CO2, a common combustion pollutant) releasing continuously into the atmosphere is primarily responsible for the rising atmospheric temperature. Therefore, CO2 sequestration has been an indispensable area of research for the past several decades. On the other hand, the concept of aromaticity is often employed in designing chemical reactions and metal‐free frustrated Lewis pairs (FLPs) have proved ideal reagents to achieve CO2 reduction. However, considering FLP and aromaticity together is less developed in CO2 capture.

Aromaticity generally describes a cyclic structure composed of sp2-hybridized carbon or hetero atoms with remarkable stability and unique reactivity. The doping of even one sp3-hybridized atom often damages the aromaticity due to the interrupted electron conjugation. Here we demonstrate the occurrence of an extended hyperconjugative aromaticity (EHA) in a metalated indole ring which contains two gem-diaurated tetrahedral carbon atoms. The EHA-involved penta-aurated indolium shows extended electron conjugation because of dual hyperconjugation.

Benzene, the prototype of aromatics, has six equivalent C‐C bonds (1.397 Å), which are intermediate between a C‐C double bond and a C‐C single bond. For over 80 years, chemists have spent much effort on freezing a localized structure to obtain a distorted bond‐length alternating benzene ring in the ground state, leading to various localized trisannelated benzene rings. However, most of the central benzene rings are still aromatic or nonaromatic. Here we report an antiaromatic benzene ring caused by hyperconjugation.
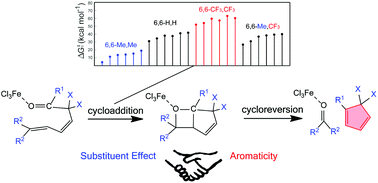
Olefin metathesis is a fundamental organic reaction of great importance that led to the 2005 Nobel Prize in Chemistry. As a variation of olefin–olefin metathesis, carbonyl–olefin metathesis (COM) is less developed, but still significant progress has been made recently. However, how the aromaticity affects the reaction mechanisms remains unclear. Here we perform density functional theory calculations on iron(III) catalyzed COM in 2,5- and 3,5-hexadienals.

Pincer complexes are a remarkably versatile family benefited from their stability, diversity, and tunability. Many of them contain aromatic organic rings at the periphery, and aromaticity plays an important role in their stability and properties, whereas their metallacyclic cores are not aromatic. Herein, we report rhodapentalenes, which can be viewed as pincer complexes in which the metallacyclic cores exhibit considerable aromatic character. Rhodapentalenes show good thermal stability, although the rhodium-carbon bonds in such compounds are fragile.
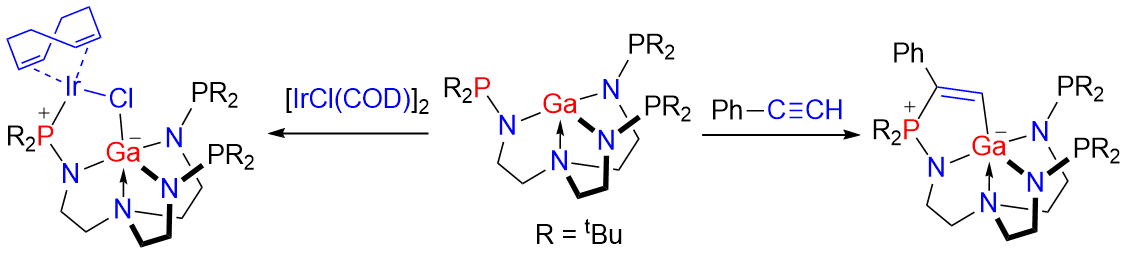
Frustrated Lewis pairs (FLPs) represent a new paradigm of main‐group chemistry. The Lewis acidic centers in FLP chemistry are typically B and Al atoms in the studies reported over the past decade, and most of them are tri‐coordinated with strong electron‐withdrawing groups. Herein, we report a Ga/P system containing an unprecedented four‐coordinated Lewis acidic Ga center. This Ga/P species performs classical addition reactions toward heterocumulenes, alkyne, diazomethane, and transition metal complex. Regioselective formation of the products can be rationalized by DFT calculations.
Copyright © 2026,
Theme Originally Created by Devsaran
