Unconventional Aromaticity in Organometallics: The Power of Transition Metals
Aromaticity, one of the most fundamental concepts in chemistry, has attracted considerable attention from both theoreticians and experimentalists. Much effort on aromaticity in organometallics has been devoted to metallabenzene and derivatives. In comparison, aromaticity in other organometallics is less developed. This Account describes how our group has performed quantum chemical calculations to examine aromaticity in recently synthesized novel organometallic complexes. By collaborations with experimentalists, we have extended several aromaticity concepts into organometallics to highlight the power of transition metals.
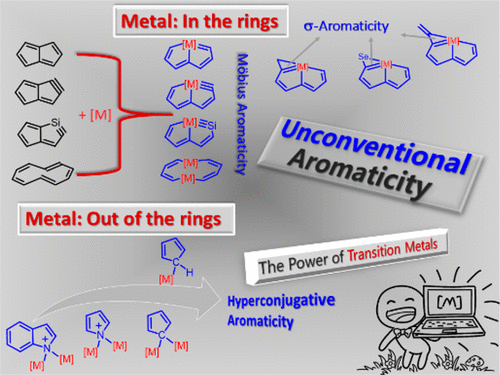
In general, the transition metal could participate in delocalization either out of rings or in the rings. We examined the former by probing the possibility of transition metal substituents in hyperconjugative aromaticity, where the metal is out of the rings. Calculations on tetraaurated heteroaryl complexes reveal that incorporation of the aurated substituents at the nitrogen atom can convert nonaromaticity in the parent indolium into aromaticity in the aurated one due to hyperconjugation, thus extending the concept of hyperconjugative aromaticity to heterocycles with transition metal substituents. More importantly, further analysis indicates that the aurated substituents can perform better than traditional main-group substituents. Recently, we also probed the strongest aromatic cyclopentadiene and pyrrolium rings by hyperconjugation of transition metal substituents. Moreover, theoretical calculations suggest that one electropositive substituent is able to induce aromaticity; whereas one electronegative substituent prompts nonaromaticity rather than antiaromaticity.
We also probed the possibility of Craig-type Möbius aromaticity in organometallic chemistry, where the position of the transition metals is in the rings. According to the electron count and topology, aromaticity can be classified as Hückel-type and Möbius-type. In comparison with numerous Hückel aromatics containing 4n+2 π-electrons, Möbius aromatics with 4n π-electrons, especially the Craig-type species, are particularly limited. We first examined aromaticity in osmapentalynes. Theoretical calculations reveal that incorporation of the osmium center not only reduces the ring strain of the parent pentalyne, but also converts Hückel antiaromaticity in the parent pentalyne into Craig-type Möbius aromaticity in metallapentalynes. Further studies show that the transition metal fragments can also make both 16e and 18e osmapentalenes aromatic, indicating that the Craig-type Möbius aromaticity in osmapentalyne is rooted in osmapentalenes. In addition, Möbius aromaticity is also possible in dimetalla[10]annulenes, where the lithium atoms are not spectator cations but play an important role due to their bonding interaction with the diene moieties.
We then examined the possibility of σ-aromaticity in an unsaturated ring. Traditional π-aromaticity is used to describe the π-conjugation in fully unsaturated rings; whereas σ-aromaticity may stabilize fully saturated rings with delocalization caused by σ-electron conjugation. We found that the unsaturated three-membered ring in cyclopropaosmapentalene is σ-aromatic. Very recently, we extended σ-aromaticity into in a fully unsaturated ring.
The concepts and examples presented here show the importance of interplay and union between experiment and theory in developing novel aromatic systems and, especially, the indispensable role of computational study in rationalization of unconventional aromaticity. All these findings highlight the strong power of transition metals originating from participation of d orbitals in aromaticity, opening an avenue to the design of unique metalla-aromatics.
