Nature Chemistry



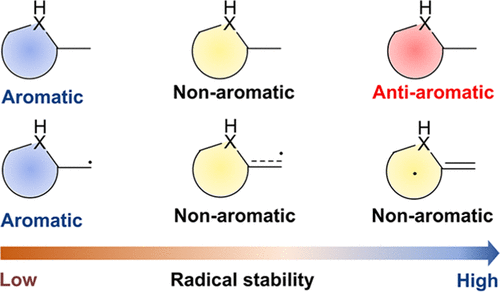
Aromaticity is a fundamental and important concept in chemistry, and usually, the enhancement of aromaticity brings additional thermodynamic stability to a compound. Moreover, since radicals can act as intermediates in chemical reactions, they have attracted considerable attention from both experimental and theoretical chemists for a long time. However, it remains unclear whether there is a relationship between the thermodynamic stability of cyclic planar radicals and their aromaticity.

Activating the C–F bond (the strongest σ bond to carbon) is particularly challenging, let alone in a selective fashion when a weaker C–H bond is present in the same species. Herein, we demonstrate a novel strategy to achieve a thermodynamically and kinetically favorable activation of the C–F bond over the C–H bond dually driven by coordination and aromaticity via density functional theory calculations.
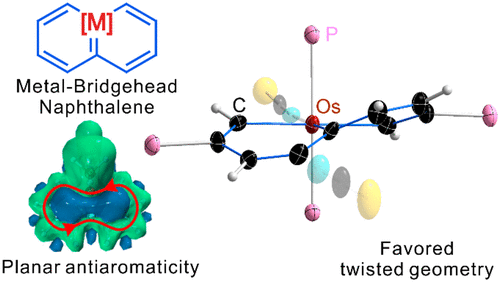
As a fundamental chemical property, aromaticity guides the synthesis of novel structures and materials. Replacing the carbon moieties of aromatic hydrocarbons with transition metal fragments is a promising strategy to synthesize intriguing organometallic counterparts with a similar aromaticity to their organic parents. However, since antiaromaticity will endow compound instability, it is a great challenge to obtain an antiaromatic organometallic counterpart based on such transition metal replacement in aromatic hydrocarbons.
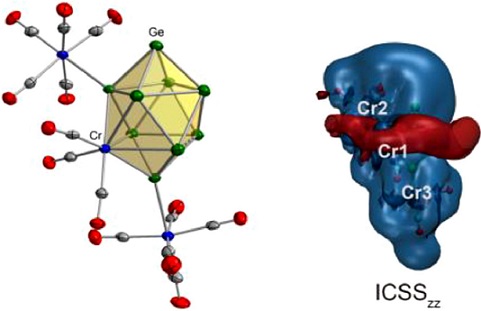
We report the first disubstituted hetero-ten-vertex closo cluster [(CrGe9)Cr2(CO)13]4- with three adjacent Cr(CO)n units adopting both η5 and η1 coordination modes, which was synthesized through the reaction of “KGe1.67” with (MeCN)3Cr(CO)3 and Cr(CO)6 in ethylenediamine (en) solution. In contrast to the η1-Cr atoms forming localized two-center two-elelctron (2c-2e) Cr-Ge bonds, the hetero atom η5-Cr exhibits versatile bonding mechanisms including three 5c-2e and five 8c-2e delocalized bonds which account for Hückel aromaticity.
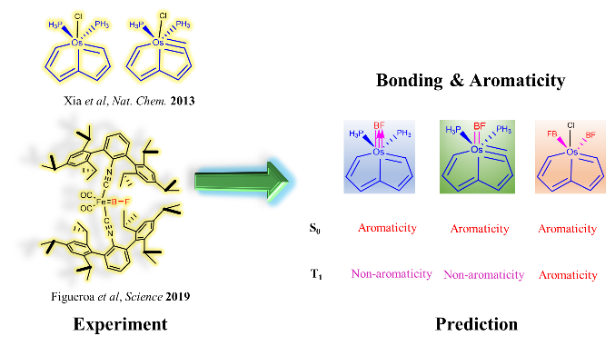
Osmapentalyne and osmapentalene complexes, now termed as carbolong species, have attracted considerable attention due to their novel structures, reactivities, chelating properties as well as Möbius and adaptive aromaticity. On the other hand, boron monofluoride (BF), a 10-electron diatomic molecule isoelectronic to carbon monoxide (CO), is unstable below 1800°C in the gas phase, and preparation of its metal complex is particularly challenging.

Due to consumption of more than 2% of the world's annual energy supply by Haber–Bosch process and the strongest triple bond (N≡N) in nature, directly coupling N 2 with small molecules is particularly important and challenging, let alone in a catalytic fashion. Here we first demonstrate that a NNN-type pincer phosphorus complex could act as a catalyst to couple dinitrogen with a series of small molecules including carbon dioxide, formaldehyde, N-ethylidenemethylamine, and acetonitrile in the presence of diborane(4) under a mild condition by theoretical calculations.
Ambiphilic reactivity is a fascinating topic in chemical reactions, attracting considerable interest because ambiphilic reagents can display properties of both nucleophilicity and electrophilicity. However, most of the previous attention has been focused on the characterization of the ambiphilic reactivity, whereas the origin is less understood. Here we carry out thorough density functional theory (DFT) calculations to probe the origin of the ambiphilic reactivity of the carbyne atom in osmapentalynes, observed previously in experiment.

As a fundamental concept in chemistry, aromaticity has been extended from traditional organics to organometallics. Similarly, hyperconjugative aromaticity (HCA) has also been developed from main group to transition metal systems through the hyperconjugation of the substituents. However, it remains unclear that how the oxidation state of transition metal in the substituents affects the HCA. Herein, we demonstrate via density functional theory calculations that HCA could disappear in indoliums when the Au(I) substituents are changed to the Au(III) ones.
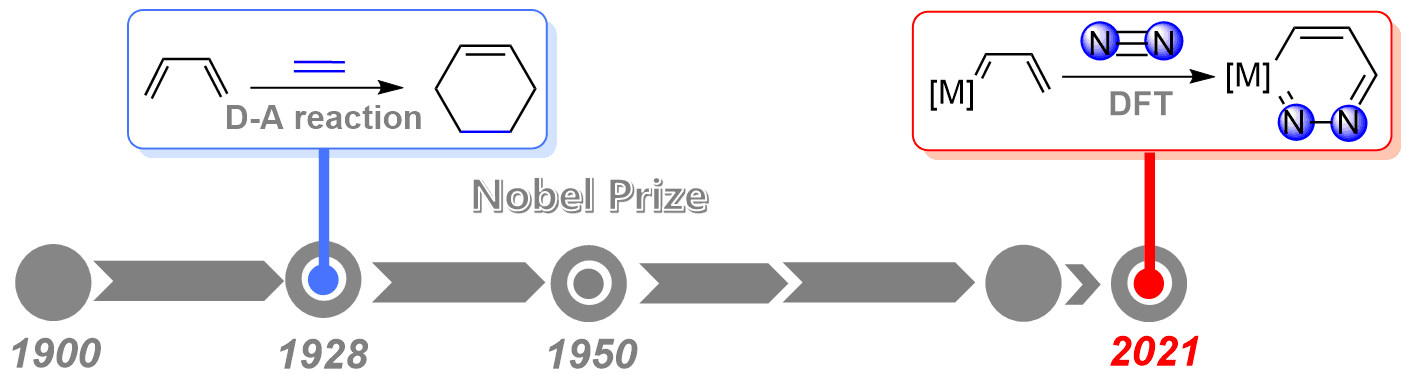
As the strongest triple bond in nature, the N≡N triple bond activation has always been a challenging project in chemistry. On the other hand, since the award of the Nobel Prize in Chemistry in 1950, Diels‐Alder reaction has served as a powerful and widely applied tool in the synthesis of natural products and new materials. However, the application of Diels‐Alder reaction to dinitrogen activation remains less developed.
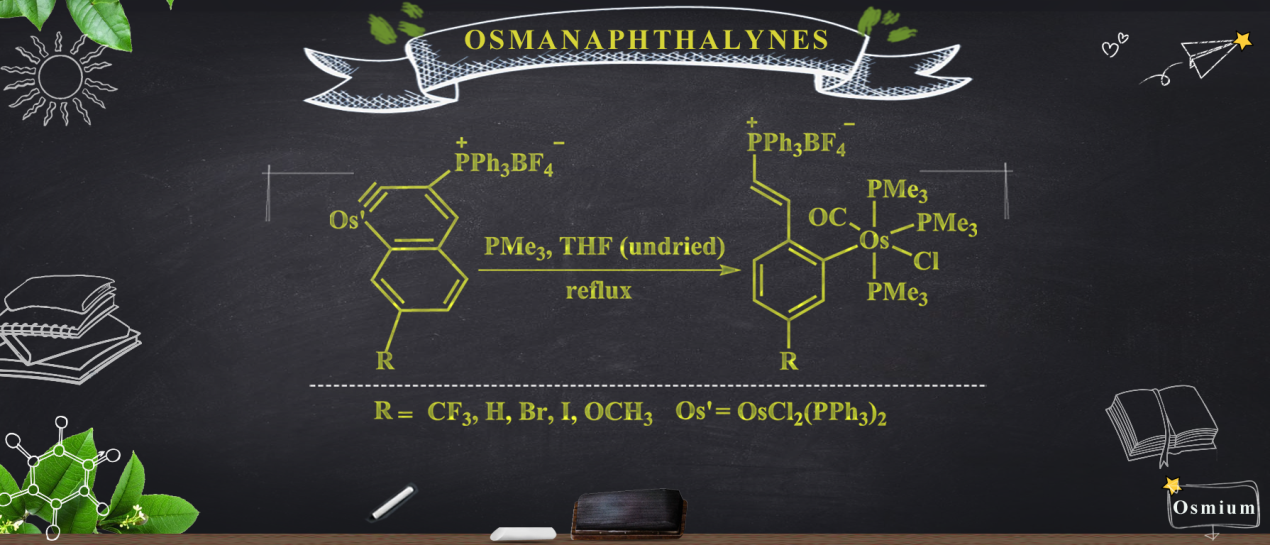
Members of a new class of complexes, 2(CF3), 2(H), 2(Br), 2(I), and 2(OCH3), have been synthesized by a one-pot method involving the treatment of osmanaphthalynes bearing corresponding substituents (1(CF3), 1(H), 1(Br), 1(I), and 1(OCH3) with trimethylphosphine (PMe3) and water (H2O).
Copyright © 2026,
Theme Originally Created by Devsaran
