Chemoselectivity for B–O and B–H Bond Cleavage by Pincer-Type Phosphorus Compounds: Theoretical and Experimental Studies
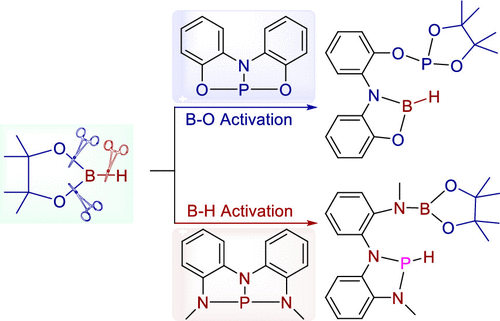
Selective cleavage of the B–O bond or B–H bond in HBpin can be achieved by adjusting the pincer ligand of a phosphorus(III) compound guided by a combination of theoretical prediction and experimental verification. Theoretical calculations reveal that a pincer-type phosphorus compound with an [ONO]3– ligand reacts with HBpin, leading to cleavage of the stronger B–O bonds (ΔG°⧧ = 23.2 kcal mol–1) rather than the weaker B–H bond (ΔG°⧧ = 26.4 kcal mol–1). A pincer-type phosphorus compound with a [NNN]3– ligand reacts with HBpin, leading to the weaker B–H bond cleavage (ΔG°⧧ = 16.2 kcal mol–1) rather than cleavage of the stronger B–O bond (ΔG°⧧ = 33.0 kcal mol–1). The theoretical prediction for B–O bond cleavage was verified experimentally, and the final products were characterized by NMR, HRMS, and single-crystal X-ray diffraction. The chemoselectivity of B–O bond cleavage was also observed in the presence of B–C or B–B bonds in borane substrates.
