Predicting Dinitrogen Coupling with a Series of Small Molecules Catalyzed by a Pincer Complex
Submitted by Jun Zhu on Wed, 06/16/2021 - 08:56
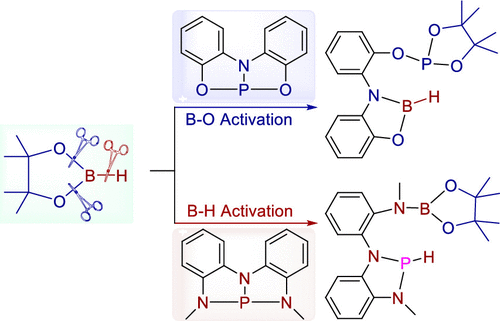
Due to consumption of more than 2% of the world's annual energy supply by Haber–Bosch process and the strongest triple bond (N≡N) in nature, directly coupling N 2 with small molecules is particularly important and challenging, let alone in a catalytic fashion. Here we first demonstrate that a NNN-type pincer phosphorus complex could act as a catalyst to couple dinitrogen with a series of small molecules including carbon dioxide, formaldehyde, N-ethylidenemethylamine, and acetonitrile in the presence of diborane(4) under a mild condition by theoretical calculations.