Open questions on aromaticity in organometallics
Submitted by Jun Zhu on Wed, 11/11/2020 - 16:44
Authors:
Jun Zhu
Journal:
Commun. Chem.
Year:
2020
Volume:
3
FirstPage-LastPage:
161
TOC:

Abstract:
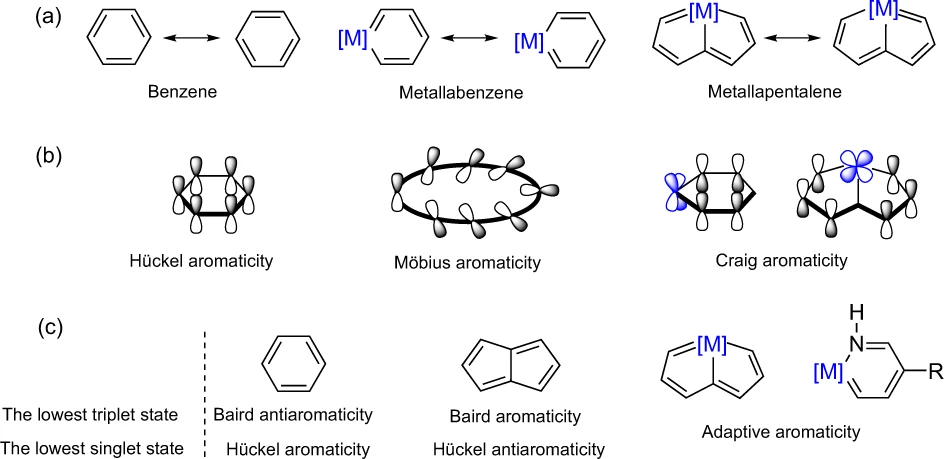
While sp2-hybridized carbon atoms in hydrocarbons typically contribute only one electron to their aromaticity, metals have more electrons from d or f orbitals available for participating in conjugation in organometallics, complicating the electron counting as well as analysis of their aromaticity. Here, the author comments on the challenges towards understanding aromaticity in organometallics and outlines several remaining questions that have yet to be answered.
Doi:
10.1038/s42004-020-00419-5
