Probing the Strongest Aromatic Cyclopentadiene Ring by Hyperconjugation
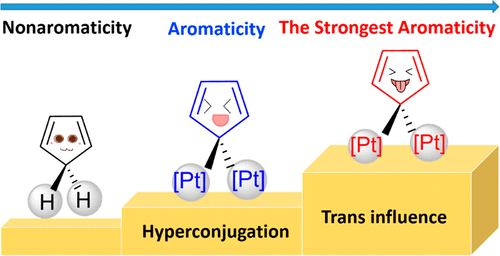
Hyperconjugation, an interaction of electrons in a σ orbital or lone pair with an adjacent π or even σ antibonding orbital, can have a strong effect on aromaticity. However, most work on hyperconjugative aromaticity has been limited to main-group substituents. Here, we report a thorough density functional theory study to evaluate the aromaticity in various cyclopentadienes that contain both main-group and transition-metal substituents. Our calculations reveal that the strongest aromatic cyclopentadiene ring can be achieved by the synergy of trans influence and hyperconjugation caused by transition-metal substituents. Our findings highlight the great power of transition metals and trans influence in achieving hyperconjugative aromaticity, opening an avenue to the design of other novel aromatic organometallics.
