Reaction Mechanisms on [3 + 2] Cycloaddition of Azides with Metal Carbyne Complexes: Significant Effects of Aromaticity, Substituent, and Metal Center
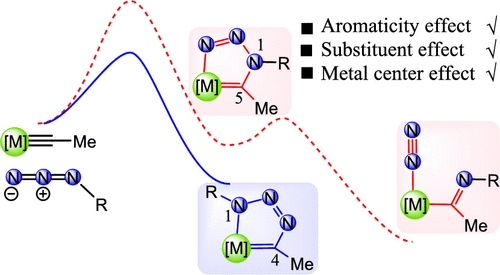
Density functional theory calculations were used to investigate the reaction mechanisms on [3 + 2] cycloaddition reactions of azides with metal carbyne complexes. Our results reveal that the formation of a 1,4-metallatriazole regioisomer is a kinetically favorable process in comparison with the formation of 1,5-metallatriazole. Aromaticity plays an important role in stabilizing the products in these reactions. Further analyses show that the electron-donating ligand on metal centers or the electron-withdrawing group on the azide could accelerate the [3 + 2] cycloaddition reaction. All of these findings could be useful for experimental chemists to develop “click reactions” in organometallic chemistry.
