Theoretical study on the stability and aromaticity in silapentafulvenes towards triplet ground state species
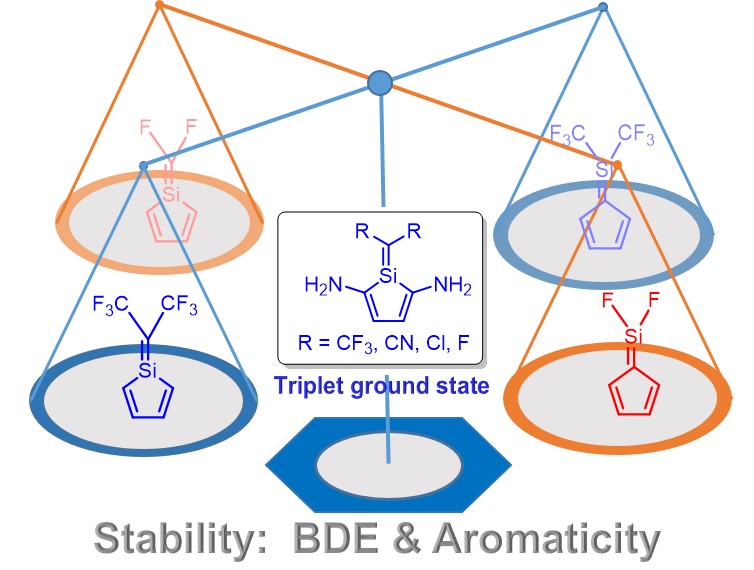
Pentafulvenes are dipolar hydrocarbons since they shift their π-electrons to achieve Hückel aromaticity and thus the electron donating groups at the exocyclic position can enhance their aromaticity. Silapentafulvenes are analogues of pentafulvene formed by the replacement of the carbon atoms at the exocyclic CC double bond with a silicon atom in pentafulvene. It remains unclear how the aromaticity of 5-silapentafulvenes and 6-silapentafulvenes can be changed due to the polarization of the CSi double bond. Here we perform density functional theory calculations and reveal the increased aromatic character in 6-silapentafulvenes and the reduced aromaticity of 5-silapentafulvenes in the ground state. In addition, the origin of the relative thermodynamic stability of the silapentafulvene isomers can be attributed to the bond dissociation energy (BDE) of the exocyclic bond. More interestingly, some triplet ground state 5-silapentafulvene species are predicted by introducing amino groups on the ring, which is supported by the coupled cluster calculations. Our findings could be useful for experimentalists to realize silaaromatics.
https://pubs.rsc.org/en/Content/ArticleLanding/2020/CP/C9CP06506G#!divAbstract
