Concerted or Stepwise Mechanism? New Insight into the Water-Mediated Neutral Hydrolysis of Carbonyl Sulfide
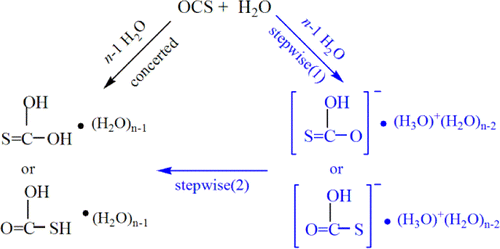
The water-mediated neutral hydrolysis mechanism of carbonyl sulfide (OCS) has been re-examined using the hybrid supramolecule/continuum models with n = 2–8 explicit water cluster at the level of MP2(fc)(CPCM)/6-311++G(d,p)//MP2(fc)(CPCM) /6-31+G(d). Present calculations indicate that the potential energy surface in water solution is different from the one in the gas-phase, and only stepwise mechanism is observed in aqueous solution, i.e., monothiocarbonic acid (H2CO2S) is formed via monothiocarbonate (OCSOH–, MTC) and its counterion, protonated water cluster, (H2O)nH3O+. The predicted rate-determining step (RDS) barrier for the stepwise mechanism in water solution, about 90 kJ/mol, shows good agreement with the experimental values, 83.7–96.2 kJ/mol using six- or eight-water model including two cooperative water molecules. Moreover, two reaction pathways, the nucleophilic addition of water molecule across the C═O or the C═S bond of OCS are competitive.
